Stavudine
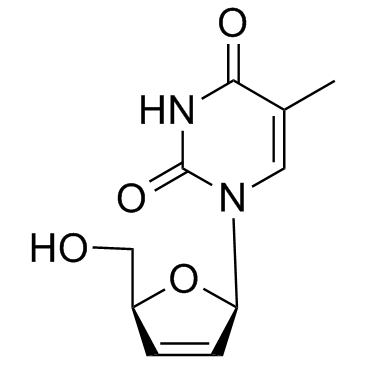
Stavudine structure
|
Common Name | Stavudine | ||
|---|---|---|---|---|
| CAS Number | 3056-17-5 | Molecular Weight | 224.213 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 440.6±55.0 °C at 760 mmHg | |
| Molecular Formula | C10H12N2O4 | Melting Point | 159-160°C | |
| MSDS | Chinese USA | Flash Point | 220.3±31.5 °C | |
Use of StavudineStavudine is a nucleoside analog that inhibits reverse transcriptase and has in vitro activity against HIV.Target: HIV RT; NRTIsStavudine is a dideoxynucleoside analog that inhibits reverse transcriptase and has in vitro activity against HIV. Stavudine is an analog of thymidine. It is phosphorylated by cellular kinases into active triphosphate. Stavudine triphosphate inhibits the HIV reverse transcriptase by competing with natural substrate, thymidine triphosphate. It also causes termination of DNA synthesis by incorporating into it [1]. Mice were treated for 2 weeks with stavudine d4T (500 mg/kg/day), L-carnitine (200 mg/kg/day) or both drugs concomitantly. Body fatness was assessed by dual energy X-ray absorptiometry, and investigations were performed in plasma, liver, muscle and WAT. D4T reduced the gain of body adiposity, WAT leptin, whole body FAO and plasma ketone bodies, and increased liver triglycerides and plasma aminotransferases with mild ultrastructural abnormalities in hepatocytes [2].Clinical indications: HIV-1 infection FDA Approved Date: June 24, 1994 Toxicity: peripheral neuropathy; lipodystrophy |
| Name | stavudine |
|---|---|
| Synonym | More Synonyms |
| Description | Stavudine is a nucleoside analog that inhibits reverse transcriptase and has in vitro activity against HIV.Target: HIV RT; NRTIsStavudine is a dideoxynucleoside analog that inhibits reverse transcriptase and has in vitro activity against HIV. Stavudine is an analog of thymidine. It is phosphorylated by cellular kinases into active triphosphate. Stavudine triphosphate inhibits the HIV reverse transcriptase by competing with natural substrate, thymidine triphosphate. It also causes termination of DNA synthesis by incorporating into it [1]. Mice were treated for 2 weeks with stavudine d4T (500 mg/kg/day), L-carnitine (200 mg/kg/day) or both drugs concomitantly. Body fatness was assessed by dual energy X-ray absorptiometry, and investigations were performed in plasma, liver, muscle and WAT. D4T reduced the gain of body adiposity, WAT leptin, whole body FAO and plasma ketone bodies, and increased liver triglycerides and plasma aminotransferases with mild ultrastructural abnormalities in hepatocytes [2].Clinical indications: HIV-1 infection FDA Approved Date: June 24, 1994 Toxicity: peripheral neuropathy; lipodystrophy |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 440.6±55.0 °C at 760 mmHg |
| Melting Point | 159-160°C |
| Molecular Formula | C10H12N2O4 |
| Molecular Weight | 224.213 |
| Flash Point | 220.3±31.5 °C |
| Exact Mass | 224.079712 |
| PSA | 84.32000 |
| LogP | -1.25 |
| Vapour Pressure | 0.0±2.4 mmHg at 25°C |
| Index of Refraction | 1.646 |
| Storage condition | −20°C |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| Water Solubility | 5-10 g/100 mL at 21 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Precursor 7 | |
|---|---|
| DownStream 9 | |
| HS Code | 2938901000 |
|---|---|
| Summary | HS: 2938901000. 4-amino-1-((2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)pyrimidin-2(1h)-one. VAT:17.0%. tax rebate rate:13.0%. supervision conditions:None. MFN tarrif:6.5%. general tariff:20.0% |
|
Both 2',3'-dideoxythymidine and its 2',3'-unsaturated derivative (2',3'-dideoxythymidinene) are potent and selective inhibitors of human immunodeficiency virus replication in vitro.
Biochem. Biophys. Res. Commun. 142 , 128, (1987) 2',3'-Dideoxythymidine (ddThd) and its 2',3'-unsaturated derivative 2',3'-dideoxythymidinene (ddeThd) are potent and selective inhibitors of human immunodeficiency virus (HIV) in vitro. When evaluated... |
|
|
The separation and detection of PET tracers via capillary electrophoresis for chemical identity and purity analysis.
J. Pharm. Biomed. Anal. 94 , 12-8, (2014) CE coupled with UV detection was assessed as a possible platform for the chemical identity and purity analysis of positron emission tomography (PET) tracers using [(18)F]FAC and [(18)F]FLT as examples... |
|
|
Viral protein R of HIV type-1 induces retrotransposition and upregulates glutamate synthesis by the signal transducer and activator of transcription 1 signaling pathway.
Microbiol. Immunol. 59 , 398-409, (2015) Viral protein R (Vpr) of HIV-1 plays an important role in viral replication in macrophages. Various lines of evidence suggest that expression of Vpr in macrophages causes immunopathogenesis; however, ... |
| 1-(2,3-Dideoxy-Beta-D-Glycero-2-Pentenofuranosyl)Thymine |
| Sanilvudine |
| Virostav |
| 2,4(1H,3H)-Pyrimidinedione, 1-[(2R,5S)-2,5-dihydro-5-(hydroxymethyl)-2-furanyl]-5-methyl- |
| 1-[(2R,5S)-5-(Hydroxymethyl)-2,5-dihydro-2-furanyl]-5-methyl-2,4(1H,3H)-pyrimidinedione |
| 4-Hydroxy-1-[(2R,5S)-5-(hydroxymethyl)-2,5-dihydro-2-furanyl]-5-methyl-2(1H)-pyrimidinone |
| Stavudine |
| Stauvidine |
| 3'-Deoxy-2'-thymidinene |
| 1-(2,3-Dideoxy-β-D-glycero-2-pentenofuranosyl)thymine |
| Zerit |
| 1-[(2R,5S)-5-(Hydroxymethyl)-2,5-dihydrofuran-2-yl]-5-methylpyrimidin-2,4(1H,3H)-dion |
| 2(1H)-Pyrimidinone, 1-[(2R,5S)-2,5-dihydro-5-(hydroxymethyl)-2-furanyl]-4-hydroxy-5-methyl- |
| 2',3'-dideoxy-2',3'-didehydrothymidine |
| 1-[(2R,5S)-5-(hydroxyméthyl)-2,5-dihydrofuran-2-yl]-5-méthylpyrimidine-2,4(1H,3H)-dione |
| Thymidine, 2',3'-didehydro-3'-deoxy- |
| 2',3'-didehydro-3'-deoxythimidine |
| 1-(2,3-Dideoxy-b-glycero-pent-2-enofuranosyl)thymine |
| D 4T |
| D4T |
| estavudina |
| MFCD00132921 |
| 2',3'-Anhydrothymidine |
| Stavir |
| Avostav |
| Sanilvudine (JAN) |
| 1-[(2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl]-5-methylpyrimidine-2,4(1H,3H)-dione |
| 2',3'-Didehydro-3'-deoxythyMidine |
| Thymine, 1-(2,3-dideoxy-β-D-glycero-pent-2-enofuranosyl)- (7CI,8CI) |
| 2',3'-dideoxy-2',3'-didehydrothymine |
![1-[5-O-(tert-butyldiphenylsilyl)-2,3-dideoxy-β-D-glycero-pento-2-enofuranosyl]thymine Structure](https://www.chemsrc.com/caspic/057/125440-17-7.png) CAS#:125440-17-7
CAS#:125440-17-7![[(2S,5R)-5-(5-methyl-2,4-dioxopyrimidin-1-yl)-2,5-dihydrofuran-2-yl]methyl acetate Structure](https://www.chemsrc.com/caspic/273/77421-68-2.png) CAS#:77421-68-2
CAS#:77421-68-2 CAS#:5964-41-0
CAS#:5964-41-0![2,5-Methano-5H,9H-pyrimido[2,1-b][1,5,3]dioxazepin-9-one,2,3-dihydro-3-(hydroxymethyl)-8-methyl-, (2R,3R,5R)- Structure](https://www.chemsrc.com/caspic/418/15981-92-7.png) CAS#:15981-92-7
CAS#:15981-92-7 CAS#:144867-91-4
CAS#:144867-91-4 CAS#:7481-90-5
CAS#:7481-90-5 CAS#:102717-29-3
CAS#:102717-29-3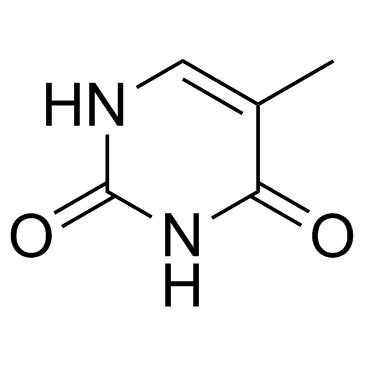 CAS#:65-71-4
CAS#:65-71-4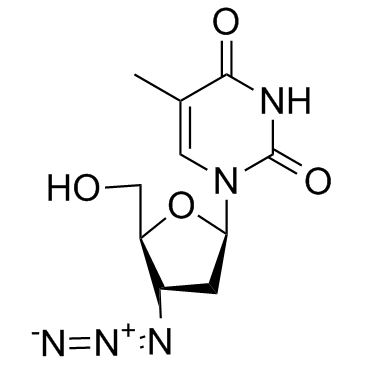 CAS#:30516-87-1
CAS#:30516-87-1![hydroxy-[[(2S,5R)-5-(5-methyl-2,4-dioxopyrimidin-1-yl)-2,5-dihydrofuran-2-yl]methoxy]-oxophosphanium structure](https://www.chemsrc.com/caspic/335/140132-35-0.png) CAS#:140132-35-0
CAS#:140132-35-0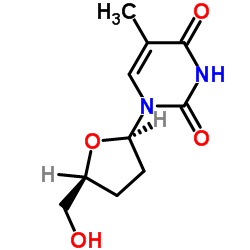 CAS#:3416-05-5
CAS#:3416-05-5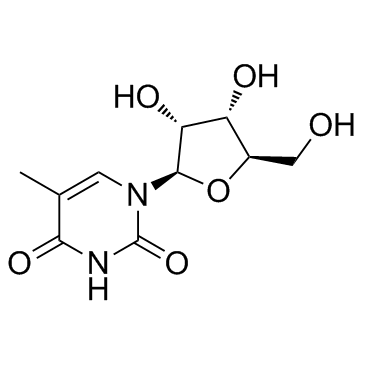 CAS#:1463-10-1
CAS#:1463-10-1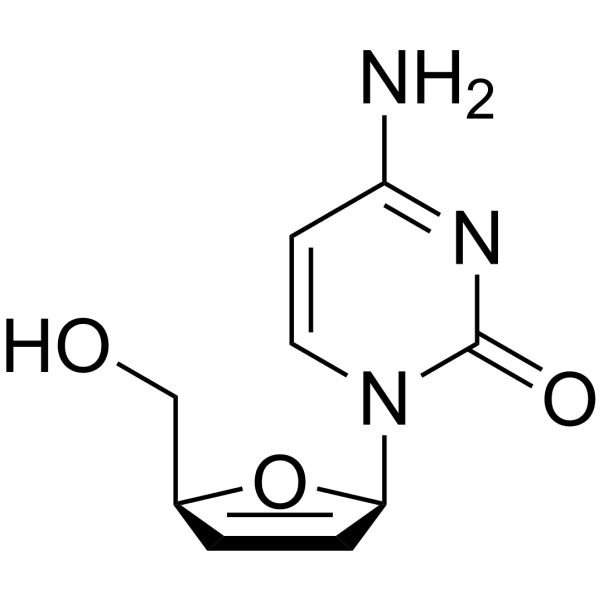 CAS#:7481-88-1
CAS#:7481-88-1![[(2S,5R)-5-(5-methyl-2,4-dioxopyrimidin-1-yl)-2,5-dihydrofuran-2-yl]methyl 2,2-dimethylpropanoate structure](https://www.chemsrc.com/caspic/441/126209-27-6.png) CAS#:126209-27-6
CAS#:126209-27-6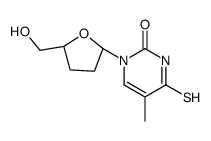 CAS#:122568-03-0
CAS#:122568-03-0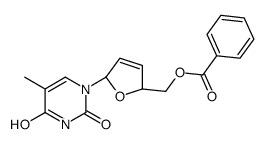 CAS#:122567-97-9
CAS#:122567-97-9
