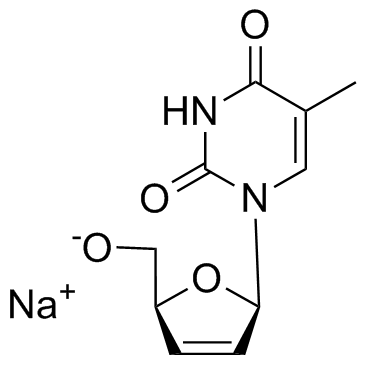
134624-73-0
| Name | Stavudine sodium |
|---|
| Description | Stavudine sodium is a nucleoside analog that inhibits reverse transcriptase and has in vitro activity against HIV.Target: HIV RT; NRTIsStavudine sodium is a dideoxynucleoside analog that inhibits reverse transcriptase and has in vitro activity against HIV. Stavudine sodium is an analog of thymidine. It is phosphorylated by cellular kinases into active triphosphate. Stavudine sodium triphosphate inhibits the HIV reverse transcriptase by competing with natural substrate, thymidine triphosphate. It also causes termination of DNA synthesis by incorporating into it [1]. Mice were treated for 2 weeks with stavudine d4T (500 mg/kg/day), L-carnitine (200 mg/kg/day) or both drugs concomitantly. Body fatness was assessed by dual energy X-ray absorptiometry, and investigations were performed in plasma, liver, muscle and WAT. D4T reduced the gain of body adiposity, WAT leptin, whole body FAO and plasma ketone bodies, and increased liver triglycerides and plasma aminotransferases with mild ultrastructural abnormalities in hepatocytes [2].Clinical indications: HIV-1 infection FDA Approved Date: June 24, 1994 Toxicity: peripheral neuropathy; lipodystrophy |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C10H11N2NaO4 |
|---|---|
| Molecular Weight | 246.2 |