Aica ribonucleotide
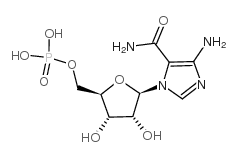
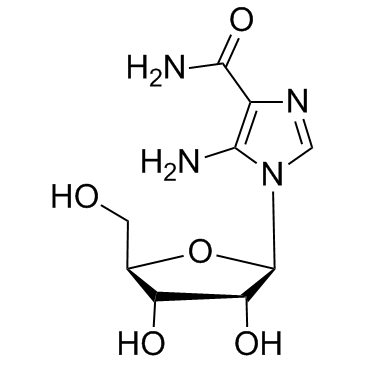
Aica ribonucleotide structure
|
Common Name | Aica ribonucleotide | ||
|---|---|---|---|---|
| CAS Number | 3031-94-5 | Molecular Weight | 338.21 | |
| Density | 2.3g/cm3 | Boiling Point | 845.3ºC at 760 mmHg | |
| Molecular Formula | C9H15N4O8P | Melting Point | 198-202ºC dec. | |
| MSDS | USA | Flash Point | 465ºC | |
Use of Aica ribonucleotideAICA-riboside, 5′-phosphate (AICAR-5'-MP) is a 5'-phosphorylated analogue of AICAR. AICAR is an adenosine analog and a AMPK activator. [1]. |
| Name | 5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranosyl 5′-monophosphate |
|---|---|
| Synonym | More Synonyms |
| Description | AICA-riboside, 5′-phosphate (AICAR-5'-MP) is a 5'-phosphorylated analogue of AICAR. AICAR is an adenosine analog and a AMPK activator. [1]. |
|---|---|
| Related Catalog |
| Density | 2.3g/cm3 |
|---|---|
| Boiling Point | 845.3ºC at 760 mmHg |
| Melting Point | 198-202ºC dec. |
| Molecular Formula | C9H15N4O8P |
| Molecular Weight | 338.21 |
| Flash Point | 465ºC |
| Exact Mass | 338.06300 |
| PSA | 213.19000 |
| Index of Refraction | 1.831 |
| InChIKey | NOTGFIUVDGNKRI-UUOKFMHZSA-N |
| SMILES | NC(=O)c1ncn(C2OC(COP(=O)(O)O)C(O)C2O)c1N |
| Storage condition | −20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | 24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 29349990 |
|
~% 
Aica ribonucleotide CAS#:3031-94-5 |
| Literature: Federation Proc., , vol. 12, p. 211 |
|
~% 
Aica ribonucleotide CAS#:3031-94-5 |
| Literature: Journal of Biological Chemistry, , vol. 228, p. 201,207 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
|
In vitro and in vivo anticancer effects of mevalonate pathway modulation on human cancer cells.
Br. J. Cancer 111(8) , 1562-71, (2014) The increasing usage of statins (the 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors) has revealed a number of unexpected beneficial effects, including a reduction in cancer risk.We investi... |
|
|
Site-directed mutagenesis of catalytic residues in N(5)-carboxyaminoimidazole ribonucleotide synthetase.
Biochemistry 52(37) , 6559-67, (2013) N(5)-CAIR synthetase, an essential enzyme in microorganisms, converts 5-aminoimidazole ribonucleotide (AIR) and bicarbonate to N(5)-CAIR with the aid of ATP. Previous X-ray crystallographic analyses o... |
|
|
Genistein suppresses LPS-induced inflammatory response through inhibiting NF-κB following AMP kinase activation in RAW 264.7 macrophages.
PLoS ONE 7(12) , e53101, (2012) Genistein, the major isoflavone in soybean, was recently reported to exert beneficial effects in metabolic disorders and inflammatory diseases. In the present study, we investigated the effects and me... |
| EINECS 221-212-1 |
| MFCD00057264 |
| 5'-AMINOIMIDAZOLE-4-CARBOXAMIDE-1-β-D-RIBOFURANOSYL 5'-MONOPHOSPHATE |