2-Nitro-5-thiocyanatobenzoic Acid
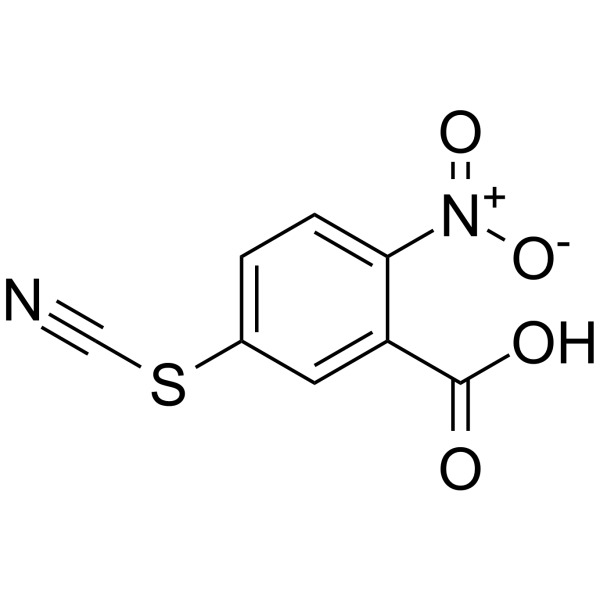
2-Nitro-5-thiocyanatobenzoic Acid structure
|
Common Name | 2-Nitro-5-thiocyanatobenzoic Acid | ||
|---|---|---|---|---|
| CAS Number | 30211-77-9 | Molecular Weight | 224.19300 | |
| Density | 1.63g/cm3 | Boiling Point | 449ºC at 760mmHg | |
| Molecular Formula | C8H4N2O4S | Melting Point | 156-157ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | 225.4ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 2-Nitro-5-thiocyanatobenzoic Acid2-Nitro-5-thiocyanatobenzoic acid (NTCB) is a highly reactive reagent that transfers its cyano group rapidly to a nucleophilic thiolate. 2-Nitro-5-thiocyanatobenzoic acid has been proposed as a reagent for converting thiol groups in proteins into their S-cyano derivatives[1][2]. |
| Name | 2-nitro-5-thiocyanatobenzoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | 2-Nitro-5-thiocyanatobenzoic acid (NTCB) is a highly reactive reagent that transfers its cyano group rapidly to a nucleophilic thiolate. 2-Nitro-5-thiocyanatobenzoic acid has been proposed as a reagent for converting thiol groups in proteins into their S-cyano derivatives[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | 2-Nitro-5-thiocyanatobenzoic acid(NTCB is a highly reactive reagent that transfers its cyano group rapidly to a nucleophilic thiolate. When it is provided to a protein, it will quickly cyanylate the protein cysteine to form S-cyano-cysteine which undergoes reversible intramolecular addition with the cysteine N-amide to generate 1-acyl-2-iminothiazolidine, an intermediate that can undergo nucleophilic acyl substitution[1]. |
| References |
| Density | 1.63g/cm3 |
|---|---|
| Boiling Point | 449ºC at 760mmHg |
| Melting Point | 156-157ºC(lit.) |
| Molecular Formula | C8H4N2O4S |
| Molecular Weight | 224.19300 |
| Flash Point | 225.4ºC |
| Exact Mass | 223.98900 |
| PSA | 132.21000 |
| LogP | 2.38938 |
| Appearance of Characters | powder | yellow |
| Index of Refraction | 1.5690 (estimate) |
| Storage condition | 2-8°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2930909090 |
| Precursor 2 | |
|---|---|
| DownStream 1 | |
| HS Code | 2930909090 |
|---|---|
| Summary | 2930909090. other organo-sulphur compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
EPOR-Based Purification and Analysis of Erythropoietin Mimetic Peptides from Human Urine by Cys-Specific Cleavage and LC/MS/MS.
J. Am. Soc. Mass Spectrom. 26 , 1617-25, (2015) The development of a new class of erythropoietin mimetic agents (EMA) for treating anemic conditions has been initiated with the discovery of oligopeptides capable of dimerizing the erythropoietin (EP... |
|
|
Probing the electrostatics of active site microenvironments along the catalytic cycle for Escherichia coli dihydrofolate reductase.
J. Am. Chem. Soc. 136(29) , 10349-60, (2014) Electrostatic interactions play an important role in enzyme catalysis by guiding ligand binding and facilitating chemical reactions. These electrostatic interactions are modulated by conformational ch... |
|
|
Nonradioactive, ultrasensitive site-specific protein-protein photocrosslinking: interactions of alpha-helix 2 of TATA-binding protein with general transcription factor TFIIA and transcriptional repressor NC2.
Nucleic Acids Res. 36(19) , 6143-54, (2008) We have developed an approach that enables nonradioactive, ultrasensitive (attamole sensitivity) site-specific protein-protein photocrosslinking, and we have applied the approach to the analysis of in... |
| NTCB |
| MFCD00007139 |
| 2-Nitro-5-thiocyanatobenzoesaeure |
| EINECS 250-093-9 |
| 2-Nitro-5-thiocyanobenzoic acid,NTCB |
| 2-Nitro-5-thiocyanobenzoic acid |
| 2-Nitro-5-thiocyano-benzoesaeure |
| Benzoic acid,2-nitro-5-thiocyanato |
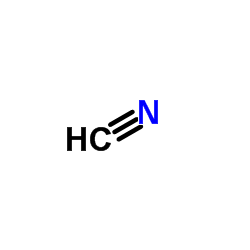 CAS#:74-90-8
CAS#:74-90-8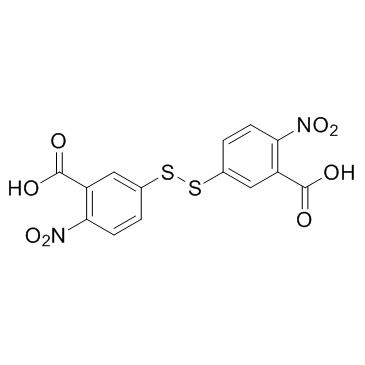 CAS#:69-78-3
CAS#:69-78-3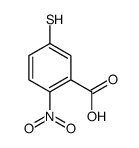 CAS#:15139-21-6
CAS#:15139-21-6