N-Benzylhydroxylamine hydrochloride
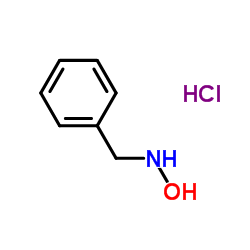
N-Benzylhydroxylamine hydrochloride structure
|
Common Name | N-Benzylhydroxylamine hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 29601-98-7 | Molecular Weight | 159.61 | |
| Density | N/A | Boiling Point | 253.9ºC at 760 mmHg | |
| Molecular Formula | C7H10ClNO | Melting Point | 108-110ºC | |
| MSDS | Chinese USA | Flash Point | 135.2ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of N-Benzylhydroxylamine hydrochlorideN-Benzylhydroxylamine hydrochloride is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | N-benzylhydroxylamine,hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | N-Benzylhydroxylamine hydrochloride is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog | |
| In Vitro | N-Benzylhydroxylamine 是一种潜在的药理学试剂,可预防和发展丙烯醛引起的视网膜色素上皮细胞损伤。 |
| Boiling Point | 253.9ºC at 760 mmHg |
|---|---|
| Melting Point | 108-110ºC |
| Molecular Formula | C7H10ClNO |
| Molecular Weight | 159.61 |
| Flash Point | 135.2ºC |
| Exact Mass | 159.045090 |
| PSA | 32.26000 |
| LogP | 2.35830 |
| InChIKey | YSNXOQGDHGUKCZ-UHFFFAOYSA-N |
| SMILES | Cl.ONCc1ccccc1 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2928000090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2928000090 |
|---|---|
| Summary | 2928000090 other organic derivatives of hydrazine or of hydroxylamine VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:20.0% |
|
Regio- and stereoselectivity of captodative olefins in 1,3-dipolar cycloadditions. A DFT/HSAB theory rationale for the observed regiochemistry of nitrones.
J. Org. Chem. 66 , 1252, (2001) Captodative olefins 1-acetylvinyl carboxylates proved to be highly regioselective dipolarophiles in 1,3-dipolar cycloadditon to propionitrile oxide, arylphenylnitrile imines, diazoalkanes, and nitrone... |
|
|
NBHA reduces acrolein-induced changes in ARPE-19 cells: possible involvement of TGFβ.
Curr. Eye Res. 36(4) , 370-8, (2011) Acrolein, a toxic, reactive aldehyde formed metabolically and environmentally, has been implicated in the damage to and dysfunction of the retinal pigment epithelium (RPE) that accompanies age-related... |
|
|
[1, 3]-Dipolar intramolecular nitrone olefin cycloaddition reaction of a sugar-derived a, ß-unsaturated ester: a new diastereo-and regioselective synthesis of an aminocyclopentitol. Jachak SM, et al.
Tetrahedron Lett. 42(29) , 4925-28, (2001)
|
| N-Benzylhydroxyamine hydrochloride |
| MFCD00043377 |
| Benzenemethanamine, N-hydroxy-, hydrochloride (1:1) |
| BnNHOH*hydrochloride |
| benzyl hydroxylamine hydrochloride |
| N-Hydroxy-1-phenylmethanamine hydrochloride (1:1) |
| o-benzylhydroxyamine hydrochloride |
| N-benzyl-N-hydroxylamine hydrochloride |
| N-Benzylhydroxylamine HCl |
| N-Benzylhydroxylamine Hydrochloride |
| N-BenzylhydroxylamineHCl |
| benzyl-hydroxylamine monohydrochloride |
| O-benzylhydroxyamine HCl |
 CAS#:3376-26-9
CAS#:3376-26-9 CAS#:622-30-0
CAS#:622-30-0 CAS#:868775-52-4
CAS#:868775-52-4 CAS#:100-46-9
CAS#:100-46-9![N-[(2-nitrophenyl)methyl]-1-phenylmethanamine Structure](https://image.chemsrc.com/caspic/197/95982-61-9.png) CAS#:95982-61-9
CAS#:95982-61-9 CAS#:128349-14-4
CAS#:128349-14-4 CAS#:103-49-1
CAS#:103-49-1 CAS#:7339-99-3
CAS#:7339-99-3 CAS#:85407-77-8
CAS#:85407-77-8 CAS#:120-51-4
CAS#:120-51-4 CAS#:27946-19-6
CAS#:27946-19-6 CAS#:79722-21-7
CAS#:79722-21-7 CAS#:932-90-1
CAS#:932-90-1 CAS#:31335-70-3
CAS#:31335-70-3 CAS#:253129-03-2
CAS#:253129-03-2 CAS#:115797-09-6
CAS#:115797-09-6
