2-Aminobenzophenone
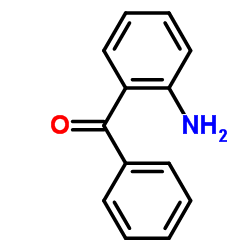
2-Aminobenzophenone structure
|
Common Name | 2-Aminobenzophenone | ||
|---|---|---|---|---|
| CAS Number | 2835-77-0 | Molecular Weight | 197.24 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 383.7±25.0 °C at 760 mmHg | |
| Molecular Formula | C13H11NO | Melting Point | 103-107 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 185.9±23.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 2-Aminobenzophenone2-Aminobenzophenone is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 2-Aminobenzophenone |
|---|---|
| Synonym | More Synonyms |
| Description | 2-Aminobenzophenone is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog | |
| In Vitro | 是有机合成、医药、农药和染料的重要原料和中间体。 |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 383.7±25.0 °C at 760 mmHg |
| Melting Point | 103-107 °C(lit.) |
| Molecular Formula | C13H11NO |
| Molecular Weight | 197.24 |
| Flash Point | 185.9±23.2 °C |
| Exact Mass | 197.084061 |
| PSA | 43.09000 |
| LogP | 2.50 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.628 |
| Water Solubility | practically insoluble |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22;R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 29223900 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2922399090 |
|---|---|
| Summary | 2922399090 other amino-aldehydes, amino-ketones and amino-quinones, other than those containing more than one kind of oxygen function; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
B-ring-aryl substituted luotonin A analogues with a new binding mode to the topoisomerase 1-DNA complex show enhanced cytotoxic activity.
PLoS ONE 9(5) , e95998, (2014) Topoisomerase 1 inhibition is an important strategy in targeted cancer chemotherapy. The drugs currently in use acting on this enzyme belong to the family of the camptothecins, and suffer severe limit... |
|
|
Effects of conformational restriction of 2-amino-3-benzoylthiophenes on A(1) adenosine receptor modulation.
J. Med. Chem. 53 , 6550-9, (2010) 2-Amino-3-benzoylthiophenes (2A3BTs) have been widely reported to act as allosteric enhancers (AEs) at the A(1) adenosine receptor (A(1)AR). Herein we describe the synthesis of a series of 1-aminoinde... |
| 2-BENZOYLANILINE |
| EINECS 220-613-9 |
| o-Benzoylaniline |
| 2-Aminobenzophenone |
| 2-Aminophenyl phenyl ketone |
| Methanone, (2-aminophenyl)phenyl- |
| 2-aminophenyl-phenylmethanone |
| 2-Benzoylbenzenamine |
| (2-Aminophenyl)(phenyl)methanone |
| MFCD00007713 |
| Benzophenone,2-amino |
| 2-amino-benzophenone |
| ortho-aminobenzophenone |
| o-Aminobenzophenone |
 CAS#:1885-29-6
CAS#:1885-29-6 CAS#:71-43-2
CAS#:71-43-2 CAS#:85-99-4
CAS#:85-99-4 CAS#:16714-27-5
CAS#:16714-27-5 CAS#:1859-71-8
CAS#:1859-71-8 CAS#:201230-82-2
CAS#:201230-82-2 CAS#:615-43-0
CAS#:615-43-0 CAS#:98-80-6
CAS#:98-80-6 CAS#:25187-00-2
CAS#:25187-00-2 CAS#:108-86-1
CAS#:108-86-1 CAS#:1072913-34-8
CAS#:1072913-34-8![N-[2-(dimethylamino)ethyl]-9-phenylacridine-4-carboxamide structure](https://image.chemsrc.com/caspic/171/112022-04-5.png) CAS#:112022-04-5
CAS#:112022-04-5 CAS#:111838-36-9
CAS#:111838-36-9 CAS#:37774-78-0
CAS#:37774-78-0 CAS#:144054-05-7
CAS#:144054-05-7 CAS#:39859-36-4
CAS#:39859-36-4 CAS#:37129-23-0
CAS#:37129-23-0 CAS#:36004-54-3
CAS#:36004-54-3 CAS#:371-40-4
CAS#:371-40-4 CAS#:1803-25-4
CAS#:1803-25-4
