Nicergoline

Nicergoline structure
|
Common Name | Nicergoline | ||
|---|---|---|---|---|
| CAS Number | 27848-84-6 | Molecular Weight | 484.385 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 594.4±50.0 °C at 760 mmHg | |
| Molecular Formula | C24H26BrN3O3 | Melting Point | 136-138° | |
| MSDS | Chinese USA | Flash Point | 313.3±30.1 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of NicergolineNicergoline is an ergot derivative used to treat senile dementia and other disorders with vascular origins.Target: Alpha-1A adrenergic receptorNicergoline acts by inhibiting the postsynaptic alpha(1)-adrenoceptors on vascular smooth muscle. This inhibits the vasoconstrictor effect of circulating and locally released catecholamines (epinephrine and norepinephrine), resulting in peripheral vasodilation. Nicergoline displaced [3H]-prazosin bound to rat forebrain membranes pretreated with chloroethylclonidine (pKi = 9.9 +/- 0.2) at concentrations 60-fold lower than in rat liver membranes (pKi = 8.1 +/- 0.2). Finally, of the nicergoline metabolites studied, lumilysergol acted as a modest alpha 1 antagonist (bromonicotinic acid was devoid of alpha 1 antagonist activity). In conclusion, nicergoline is a potent and selective alpha 1A-adrenoceptor subtype antagonist, an alpha 1-adrenoceptor subtype which is mainly represented in resistance arteries [1]. |
| Name | Nicergoline |
|---|---|
| Synonym | More Synonyms |
| Description | Nicergoline is an ergot derivative used to treat senile dementia and other disorders with vascular origins.Target: Alpha-1A adrenergic receptorNicergoline acts by inhibiting the postsynaptic alpha(1)-adrenoceptors on vascular smooth muscle. This inhibits the vasoconstrictor effect of circulating and locally released catecholamines (epinephrine and norepinephrine), resulting in peripheral vasodilation. Nicergoline displaced [3H]-prazosin bound to rat forebrain membranes pretreated with chloroethylclonidine (pKi = 9.9 +/- 0.2) at concentrations 60-fold lower than in rat liver membranes (pKi = 8.1 +/- 0.2). Finally, of the nicergoline metabolites studied, lumilysergol acted as a modest alpha 1 antagonist (bromonicotinic acid was devoid of alpha 1 antagonist activity). In conclusion, nicergoline is a potent and selective alpha 1A-adrenoceptor subtype antagonist, an alpha 1-adrenoceptor subtype which is mainly represented in resistance arteries [1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 594.4±50.0 °C at 760 mmHg |
| Melting Point | 136-138° |
| Molecular Formula | C24H26BrN3O3 |
| Molecular Weight | 484.385 |
| Flash Point | 313.3±30.1 °C |
| Exact Mass | 483.115753 |
| PSA | 56.59000 |
| LogP | 4.34 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.670 |
| InChIKey | YSEXMKHXIOCEJA-FVFQAYNVSA-N |
| SMILES | COC12CC(COC(=O)c3cncc(Br)c3)CN(C)C1Cc1cn(C)c3cccc2c13 |
| Storage condition | 2-8°C |
| Water Solubility | Practically insoluble in water, freely soluble in methylene chloride, soluble in ethanol (96 per cent). | Soluble in alcohol, chloroform, and acetone. Insoluble in water. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22 |
| Safety Phrases | S36 |
| RIDADR | UN 1544 |
| WGK Germany | 3 |
| RTECS | KE6341000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| Precursor 0 | |
|---|---|
| DownStream 2 | |
|
Structural alteration of cell surface heparan sulfate through the stimulation of the signaling pathway for heparan sulfate 6-O-sulfotransferase-1 in mouse fibroblast cells.
Biosci. Biotechnol. Biochem. 78(5) , 770-9, (2014) Heparan sulfate (HS) is a randomly sulfated polysaccharide that is present on the cell surface and in the extracellular matrix. The sulfated structures of HS were synthesized by multiple HS sulfotrans... |
|
|
[Nicergoline, ibudilast, ifenprodil tartrate].
Nihon Rinsho. 64 Suppl 7 , 602-5, (2006)
|
|
|
Protective effects of nicergoline against neuronal cell death induced by activated microglia and astrocytes.
Brain Res. 1066(1-2) , 78-85, (2005) We examined the neuroprotective role of nicergoline in neuron-microglia or neuron-astrocytes co-cultures. Nicergoline, an ergoline derivative, significantly suppressed the neuronal cell death induced ... |
| Ergobel |
| Nicergoline |
| Sermion |
| MNE |
| Cergodum |
| fi6714 |
| nigergoline |
| 3-Pyridinecarboxylic acid, 5-bromo-, [(8β)-10-methoxy-1,6-dimethylergolin-8-yl]methyl ester |
| Dilasenil |
| Cholergol |
| [(8β)-10-Methoxy-1,6-dimethylergolin-8-yl]methyl 5-bromonicotinate |
| Nargoline |
| 5-Bromopyridine-3-carboxylic Acid [(8R,10S)-10-Methoxy-1,6-dimethylergolin-8-yl]methyl Ester |
| 10-Methoxy-1,6-dimethylergoline-8β-methanol 5-bromonicotinate ester |
| TA 079 |
| (8b)-10-Methoxy-1,6-dimethylergoline-8-methanol 5-Bromo-3-pyridinecarboxylate (Ester) |
| 4,6,6a,7,8,9,10,10a-Octahydro-10aa-methoxy-4,7-dimethylindolo[4,3-fg]quinoline-9-methanol 5-Bromonicotinate |
| 5-bromo-nicotinic acid 10-methoxy-1,6-dimethyl-ergolin-8-ylmethyl ester |
| 1-Methyllumilysergol 8-(5-Bromonicotinate) 10-Methyl Ether |
| ergoline-8-methanol 10-methoxy-1,6-dimethyl-,8-(5-bromo-3-pyridinecarboxylate) |
| [(8R,10S)-10-Methoxy-1,6-dimethylergolin-8-yl]methyl 5-Bromopyridine-3-carboxylate |
| Nicergoline [USAN:BAN:INN:JAN] |
| Ergotop |
| [(8β)-10-methoxy-1,6-dimethylergolin-8-yl]methyl 5-bromopyridine-3-carboxylate |
| Memoq |
| 8b-[(5-Bromonicotinoyloxy)methyl]-1,6-dimethyl-10a-methoxyergoline |
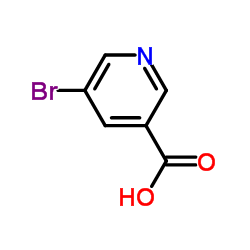 CAS#:20826-04-4
CAS#:20826-04-4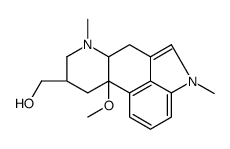 CAS#:35155-28-3
CAS#:35155-28-3
