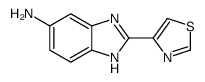
Cambendazol
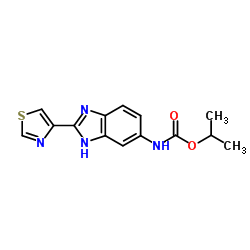
Cambendazol structure
|
Common Name | Cambendazol | ||
|---|---|---|---|---|
| CAS Number | 26097-80-3 | Molecular Weight | 302.352 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C14H14N4O2S | Melting Point | 212-214℃ (ethyl acetate hexane ) | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS08 |
Signal Word | Warning | |
Use of CambendazolCambendazole is one of the most effective agents for the therapy of human strongyloidiasis and [1]. |
| Name | propan-2-yl N-[2-(1,3-thiazol-4-yl)-3H-benzimidazol-5-yl]carbamate |
|---|---|
| Synonym | More Synonyms |
| Description | Cambendazole is one of the most effective agents for the therapy of human strongyloidiasis and [1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Melting Point | 212-214℃ (ethyl acetate hexane ) |
| Molecular Formula | C14H14N4O2S |
| Molecular Weight | 302.352 |
| Exact Mass | 302.083740 |
| PSA | 108.14000 |
| LogP | 2.90 |
| Index of Refraction | 1.694 |
| InChIKey | QZWHWHNCPFEXLL-UHFFFAOYSA-N |
| SMILES | CC(C)OC(=O)Nc1ccc2nc(-c3cscn3)[nH]c2c1 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
|
~% 
Cambendazol CAS#:26097-80-3 |
| Literature: Merck and Co., Inc. Patent: US3956488 A1, 1976 ; |
|
~% 
Cambendazol CAS#:26097-80-3 |
| Literature: Rajappa, S.; Sreenivasan, R. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, 1980 , vol. 19, # 7 p. 539 - 541 |
|
~% 
Cambendazol CAS#:26097-80-3 |
| Literature: Rajappa, S.; Sreenivasan, R. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, 1980 , vol. 19, # 7 p. 539 - 541 |
|
~% 
Cambendazol CAS#:26097-80-3 |
| Literature: Rajappa, S.; Sreenivasan, R. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, 1980 , vol. 19, # 7 p. 539 - 541 |
|
The Hymenolepis diminuta-golden hamster (Mesocricetus auratus) model for the evaluation of gastrointestinal anticestode activity.
J. Parasitol. 90(4) , 898-9, (2004) A novel laboratory anticestode assay was developed using Hymenolepis diminuta in the hamster. The commercial anticestode compounds, praziquantel, bunamidine, and niclosamide were active against patent... |
|
|
Structural requirements for the induction of cytochromes P450 by benzimidazole anthelmintic derivatives in cultured rabbit hepatocytes.
Biochem. Biophys. Res. Commun. 220(3) , 789-94, (1996) The effect of sulfur-containing benzimidazoles (thiabendazole, 5-hydroxy-thiabendazole, cambendazole) and sulfur-free derivatives (benzimidazole, carbendazim and 5-hydroxycarbendazim) on cytochrome P4... |
|
|
Controlled tests of activity of several antiparasitic compounds against natural infections of Haemonchus contortus and other helminths in lambs from a flock established in 1962.
Am. J. Vet. Res. 54(3) , 406-10, (1993) Antiparasitic activity of several compounds was evaluated over a long period (about 25 years) in the same flock of sheep. Haemonchus contortus was of special interest, including its relation to drug r... |
| Cambenzole |
| Carbamic acid, N-[2-(4-thiazolyl)-1H-benzimidazol-6-yl]-, 1-methylethyl ester |
| Novazole |
| Isopropyl [2-(1,3-thiazol-4-yl)-1H-benzimidazol-5-yl]carbamate |
| Equiben |
| Camvet |
| (2-thiazol-4-yl-1(3)H-benzoimidazol-5-yl)-carbamic acid isopropyl ester |
| CBDZ |
| propan-2-yl [2-(1,3-thiazol-4-yl)-1H-benzimidazol-5-yl]carbamate |
| cambendazole |
| Carbamic acid, (2-(4-thiazolyl)-1H-benzimidazol-5-yl)-, 1-methylethyl ester |
| Isopropyl 2-(4-thiazolyl)-5-benzimidazolecarbamate |
| NOE [French] |
| Noviben |
| Bonlam |
| [2-(4-thiazolyl)-1H-benzimidazol-5-yl]carbamic acid 1-methylethyl ester |
| 5-isopropoxycarbonylamino-2-(4'-thiazolyl)-benzimidazole |
| Camdan |
| Cambendazol |