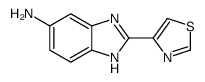
26097-80-3
| Name | propan-2-yl N-[2-(1,3-thiazol-4-yl)-3H-benzimidazol-5-yl]carbamate |
|---|---|
| Synonyms |
Cambenzole
Carbamic acid, N-[2-(4-thiazolyl)-1H-benzimidazol-6-yl]-, 1-methylethyl ester Novazole Isopropyl [2-(1,3-thiazol-4-yl)-1H-benzimidazol-5-yl]carbamate Equiben Camvet (2-thiazol-4-yl-1(3)H-benzoimidazol-5-yl)-carbamic acid isopropyl ester CBDZ propan-2-yl [2-(1,3-thiazol-4-yl)-1H-benzimidazol-5-yl]carbamate cambendazole Carbamic acid, (2-(4-thiazolyl)-1H-benzimidazol-5-yl)-, 1-methylethyl ester Isopropyl 2-(4-thiazolyl)-5-benzimidazolecarbamate NOE [French] Noviben Bonlam [2-(4-thiazolyl)-1H-benzimidazol-5-yl]carbamic acid 1-methylethyl ester 5-isopropoxycarbonylamino-2-(4'-thiazolyl)-benzimidazole Camdan Cambendazol |
| Description | Cambendazole is one of the most effective agents for the therapy of human strongyloidiasis and [1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Melting Point | 212-214℃ (ethyl acetate hexane ) |
| Molecular Formula | C14H14N4O2S |
| Molecular Weight | 302.352 |
| Exact Mass | 302.083740 |
| PSA | 108.14000 |
| LogP | 2.90 |
| Index of Refraction | 1.694 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H361 |
| Precautionary Statements | P281 |
| Hazard Codes | Xn |
| Risk Phrases | 63 |
| Safety Phrases | 36/37 |
| RIDADR | NONH for all modes of transport |
| RTECS | DD6538000 |
|
~% 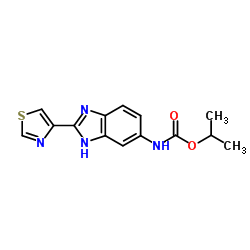
26097-80-3 |
| Literature: Merck and Co., Inc. Patent: US3956488 A1, 1976 ; |
|
~% 
26097-80-3 |
| Literature: Rajappa, S.; Sreenivasan, R. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, 1980 , vol. 19, # 7 p. 539 - 541 |
|
~% 
26097-80-3 |
| Literature: Rajappa, S.; Sreenivasan, R. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, 1980 , vol. 19, # 7 p. 539 - 541 |
|
~% 
26097-80-3 |
| Literature: Rajappa, S.; Sreenivasan, R. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, 1980 , vol. 19, # 7 p. 539 - 541 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |