5Z-7-Oxozeaenol
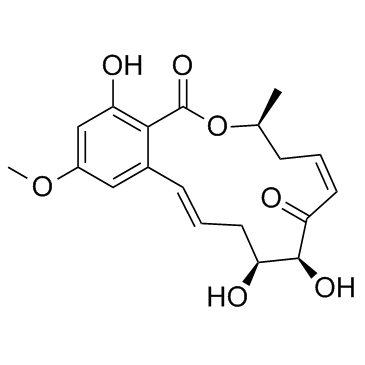
5Z-7-Oxozeaenol structure
|
Common Name | 5Z-7-Oxozeaenol | ||
|---|---|---|---|---|
| CAS Number | 253863-19-3 | Molecular Weight | 362.37400 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C19H22O7 | Melting Point | N/A | |
| MSDS | USA | Flash Point | N/A | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of 5Z-7-Oxozeaenol5Z-7-Oxozeaenol is a natural anti-protozoan compound from fungal origin, acting as a potent irreversible and selective inhibitor of TAK1 and VEGF-R2, with IC50s of 8 nM and 52 nM, respectively. |
| Name | (2E,5S,6S,8Z,11S)-5,6,15-trihydroxy-17-methoxy-11-methyl-12-oxabicyclo[12.4.0]octadeca-1(14),2,8,15,17-pentaene-7,13-dione |
|---|---|
| Synonym | More Synonyms |
| Description | 5Z-7-Oxozeaenol is a natural anti-protozoan compound from fungal origin, acting as a potent irreversible and selective inhibitor of TAK1 and VEGF-R2, with IC50s of 8 nM and 52 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
TAK1:8.1 nM (IC50) MEK1:411 nM (IC50) VEGFR-2:52 nM (IC50) VEGFR-3:110 nM (IC50) FLT3:170 nM (IC50) PDGFR-β:340 nM (IC50) B-RAF VE:6300 nM (IC50) SRC:6600 nM (IC50) |
| In Vitro | 5Z-7-Oxozeaenol is a potent irreversible and selective inhibitor of transforming growth factor (TGF)-β-activated kinase 1 (TAK1, IC50, 8.1 nM), less active on MEK1 (IC50, 411 nM). 5Z-7-Oxozeaenol prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase[1]. 5Z-7-Oxozeaenol is also an inhibitor of VEGF-R2, with an IC50 of 52 nM. 5Z-7-Oxozeaenol has inhibitory activity against VEGF-R3, FLT3, PDGFR-β, B-RAF VE and SRC, with IC50s of 110, 170, 340, 6300 and 6600 nM, respectively[2]. 5Z-7-Oxozeaenol inhibits JNK/p38 paythway, but it is not a direct inhibitor and is signal specific. 5Z-7-Oxozeaenol suppresses the PMA-induced AP-1 activity almost to the basal level in the KT cells, but has no effects on IL-1-induced NF-kB activity in the KK cells[3]. |
| Kinase Assay | For screening TAK1 inhibitors, insect expression vectors for TAK1 and TAB1 are co-infected into Sf9 cells. After 2 days of incubation, cell lysates are immunoprecipitated with anti-TAK1 antibody (M-17). The immunoprecipitates are incubated with various compounds (5Z-7-Oxozeaenol, etc.) and subsequently incubated with 2 μg of myelin basic protein and 10 μCi of [γ-32P]ATP (3,000 Ci/mmol) in 10 μL of the kinase buffer containing 10 mM HEPES (pH 7.4), 1 mM dithiothreitol, 5 mM MgCl2 at 30°C for 5 min. Samples are separated by 10% SDS-PAGE, and 32P incorporated into myelin basic protein is quantified with a bioimage analyzer. The catalytic activity of MEK1 is determined by activation of ERK2 to phosphorylate dure. The catalytic activity of MEKK1 is measured with 2 μg of myelin basic protein as a substrate in the kinase buffer. For subsequent kinase assays, immunoprecipitates are incubated with 5 μCi of [γ-32P]ATP (3,000 Ci/mmol) and 1 μg of bacterially expressed MKK6 or GST-IκBα-(1-72) in 10 μL of the kinase buffer at 25°C for 2 min. Samples are separated by 10% SDS-PAGE and visualized by autoradiography[1]. |
| References |
| Molecular Formula | C19H22O7 |
|---|---|
| Molecular Weight | 362.37400 |
| Exact Mass | 362.13700 |
| PSA | 113.29000 |
| LogP | 1.60020 |
| Storage condition | 2-8℃ |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| RIDADR | UN 2811 6.1 / PGIII |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
|
Docosahexaenoic acid and eicosapentaenoic acid suppress adhesion molecule expression in human aortic endothelial cells via differential mechanisms.
Mol. Nutr. Food. Res. 59(4) , 751-62, (2015) Dietary PUFAs modulate the progression of cardiovascular disease, but the underlying mechanisms within vascular cells remain unclear. The aim of this study was to investigate the biological function a... |
|
|
Flavonoids inhibit COX-1 and COX-2 enzymes and cytokine/chemokine production in human whole blood.
Inflammation 38(2) , 858-70, (2015) Cyclooxygenase 2 (COX-2) and the production of cytokines/chemokines are important targets for the modulation of the inflammatory response. Although a large variety of inhibitors of these pathways have... |
|
|
Disruption of thioredoxin metabolism enhances the toxicity of transforming growth factor β-activated kinase 1 (TAK1) inhibition in KRAS-mutated colon cancer cells.
Redox Biol. 5 , 319-27, (2016) Transforming growth factor β-activated kinase 1 (TAK1) is critical for survival of many KRAS mutated colorectal cancer cells, and TAK1 inhibition with 5Z-7-oxozeaenol has been associated with oxidativ... |
| (3s,5z,8s,9s,11e)-8,9,16-Trihydroxy-14-Methoxy-3-Methyl-3,4,9,10-Tetrahydro-1h-2-Benzoxacyclotetradecine-1,7(8h)-Dione |
| LL-Z1640-2 |
| AmbotzLS-1148 |
| 5Z-7-Oxozeaenol |
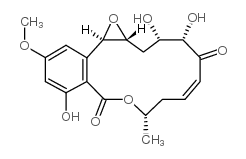 CAS#:76958-67-3
CAS#:76958-67-3