4-Chloroindole

4-Chloroindole structure
|
Common Name | 4-Chloroindole | ||
|---|---|---|---|---|
| CAS Number | 25235-85-2 | Molecular Weight | 151.593 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 293.0±13.0 °C at 760 mmHg | |
| Molecular Formula | C8H6ClN | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 158.9±5.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 4-Chloroindole |
| Name | 4-Chloroindole |
|---|---|
| Synonym | More Synonyms |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 293.0±13.0 °C at 760 mmHg |
| Molecular Formula | C8H6ClN |
| Molecular Weight | 151.593 |
| Flash Point | 158.9±5.4 °C |
| Exact Mass | 151.018875 |
| PSA | 15.79000 |
| LogP | 2.74 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.688 |
| Storage condition | 2-8°C |
| Stability | Store in Refrigerator |
| Water Solubility | insoluble |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R20/21/22;R36/37/38 |
| Safety Phrases | S26-S36-S36/37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| Precursor 7 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
The stability of the nitrosated products of indole, indole-3-acetonitrile, indole-3-carbinol and 4-chloroindole.
Food Chem. Toxicol. 27(11) , 723-30, (1989) The nitrosation rates of indole-3-acetonitrile, indole-3-carbinol, indole and 4-chloroindole and the stability of their nitrosated products were investigated. Each of the nitrosated indole compounds w... |
|
|
In-vitro testing and the carcinogenic potential of several nitrosated indole compounds.
Cell Biol. Toxicol. 7(4) , 371-86, (1991) 4-chloro-methoxyindole is a naturally occurring compound in Vicia faba which can easily react with nitrite to form a N-nitroso compound. In this in vitro study, the potential genotoxic effects of nitr... |
|
|
Expansion of substrate specificity of cytochrome P450 2A6 by random and site-directed mutagenesis.
J. Biol. Chem. 280(49) , 41090-100, (2005) The natural product indole is a substrate for cytochrome P450 2A6. Mutagenesis of P450 2A6 was done to expand its capability in the oxidization of bulky substituted indole compounds, which are not sub... |
| 4-Chloroindole |
| 1H-Indole, 4-chloro- |
| 1H-Indole,4-chloro |
| EINECS 246-747-8 |
| 4-Chloro-1H-indole |
| Indole,4-chloro |
| MFCD00005665 |
| 4-chloro-indole |
 CAS#:100376-53-2
CAS#:100376-53-2 CAS#:83-42-1
CAS#:83-42-1![3-chloro-trans-2-[β-(dimethylamino)vinyl]-nitrobenzene Structure](https://image.chemsrc.com/caspic/107/32991-06-3.png) CAS#:32991-06-3
CAS#:32991-06-3 CAS#:4637-24-5
CAS#:4637-24-5 CAS#:102493-68-5
CAS#:102493-68-5 CAS#:24621-73-6
CAS#:24621-73-6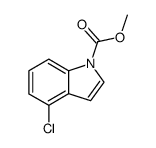 CAS#:81038-36-0
CAS#:81038-36-0 CAS#:475102-11-5
CAS#:475102-11-5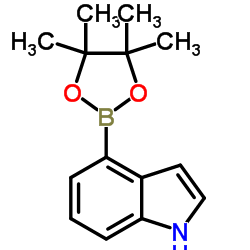 CAS#:388116-27-6
CAS#:388116-27-6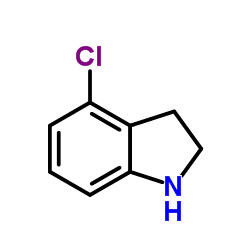 CAS#:41910-64-9
CAS#:41910-64-9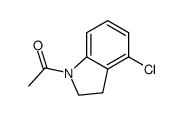 CAS#:860024-86-8
CAS#:860024-86-8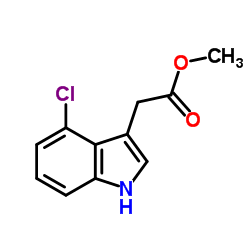 CAS#:19077-78-2
CAS#:19077-78-2 CAS#:196881-05-7
CAS#:196881-05-7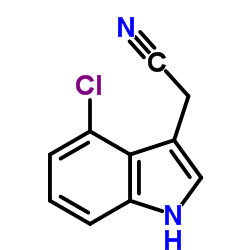 CAS#:2447-15-6
CAS#:2447-15-6 CAS#:876-72-2
CAS#:876-72-2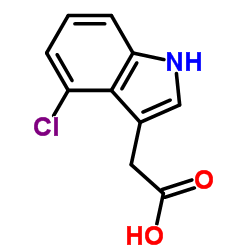 CAS#:2519-61-1
CAS#:2519-61-1 CAS#:102855-24-3
CAS#:102855-24-3
