Phenylethyl isothiocyanate
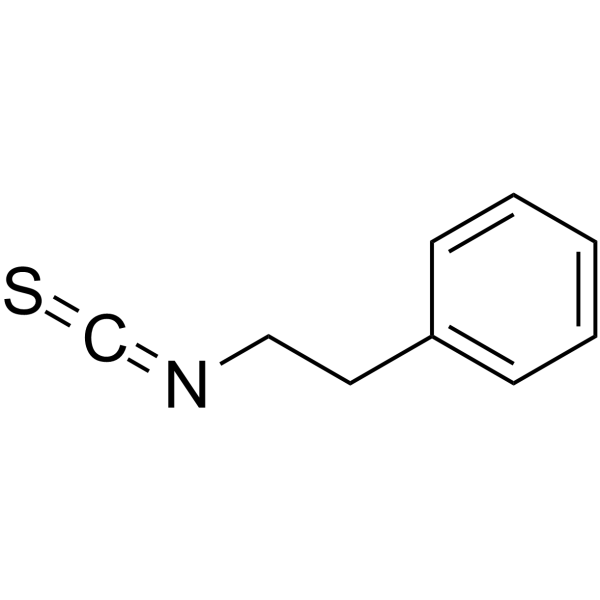
Phenylethyl isothiocyanate structure
|
Common Name | Phenylethyl isothiocyanate | ||
|---|---|---|---|---|
| CAS Number | 2257-09-2 | Molecular Weight | 163.23900 | |
| Density | 1.094 g/mL at 25 °C(lit.) | Boiling Point | 75 °C0.25 mm Hg | |
| Molecular Formula | C9H9NS | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | >230 °F | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
Use of Phenylethyl isothiocyanate2-Phenylethyl isothiocyanate is a potent antifungal agent. 2-Phenylethyl isothiocyanate significantly inhibited spore germination and mycelial growth of Alternaria alternata, with a MIC (minimum inhibitory concentration) of 1.22 mM. The antifungal effect of 2-Phenylethyl isothiocyanate against Alternaria alternata might be via reduction in toxin content and breakdown of cell membrane integrity[1][2]. |
| Name | phenethyl isothiocyanate |
|---|---|
| Synonym | More Synonyms |
| Description | 2-Phenylethyl isothiocyanate is a potent antifungal agent. 2-Phenylethyl isothiocyanate significantly inhibited spore germination and mycelial growth of Alternaria alternata, with a MIC (minimum inhibitory concentration) of 1.22 mM. The antifungal effect of 2-Phenylethyl isothiocyanate against Alternaria alternata might be via reduction in toxin content and breakdown of cell membrane integrity[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.094 g/mL at 25 °C(lit.) |
|---|---|
| Boiling Point | 75 °C0.25 mm Hg |
| Molecular Formula | C9H9NS |
| Molecular Weight | 163.23900 |
| Flash Point | >230 °F |
| Exact Mass | 163.04600 |
| PSA | 44.45000 |
| LogP | 2.33190 |
| Vapour Pressure | 0.0427mmHg at 25°C |
| Index of Refraction | n20/D 1.5888(lit.) |
| Water Solubility | insoluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302 + H312 + H332-H315-H317-H319-H334-H335 |
| Precautionary Statements | P261-P280-P305 + P351 + P338-P342 + P311 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R20/21/22 |
| Safety Phrases | S23-S26-S36-S36/37 |
| RIDADR | UN 2206 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | NX9115000 |
| Packaging Group | III |
| Hazard Class | 8 |
| HS Code | 2930909090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2930909090 |
|---|---|
| Summary | 2930909090. other organo-sulphur compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Phenethyl isothiocyanate, a naturally occurring phytochemical, is an antagonist of the aryl hydrocarbon receptor.
Mol. Nutr. Food. Res. 56(3) , 425-34, (2012) The aryl hydrocarbon (Ah) receptor is a ligand-activated transcription factor that is activated by many carcinogens, and its target gene products play a major role in tumour development, so that antag... |
|
|
Prediction and identification of drug interactions with the human ATP-binding cassette transporter multidrug-resistance associated protein 2 (MRP2; ABCC2).
J. Med. Chem. 51 , 3275-87, (2008) The chemical space of registered oral drugs was explored for inhibitors of the human multidrug-resistance associated protein 2 (MRP2; ABCC2), using a data set of 191 structurally diverse drugs and dru... |
|
|
In vitro metabolic conversion of the organic breakdown products of glucosinolate to goitrogenic thiocyanate anion.
J. Sci. Food Agric. 95 , 2244-51, (2015) Glucosinolates are abundant in Brassicaceae vegetables, and they are degraded into various organic breakdown products (BPs) (R-CN, -NCS and -SCN) by myrosinase when plant tissues are damaged. This stu... |
| PhCH2CH2NCS |
| Phenylethyl isothiocyanate |
| 2-Phenylethyl Isothiocyanate |
| EINECS 218-855-5 |
| PEITC |
| Isothiocyanic Acid 2-Phenylethyl Ester |
| Phenylaethylsenfoel |
| Phenethyl mustard oil |
| MFCD00004821 |
| Phenethyl Isothiocyanate |
| 2-phenylethyl-ITC |
| 2-isothiocyanatoethylbenzene |
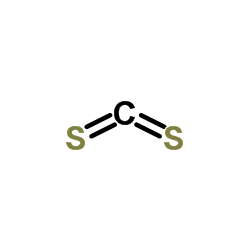 CAS#:75-15-0
CAS#:75-15-0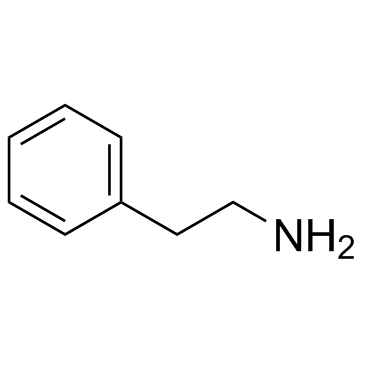 CAS#:64-04-0
CAS#:64-04-0 CAS#:24954-65-2
CAS#:24954-65-2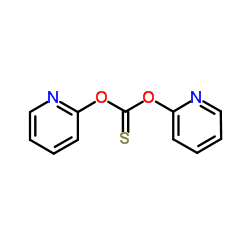 CAS#:96989-50-3
CAS#:96989-50-3 CAS#:19457-11-5
CAS#:19457-11-5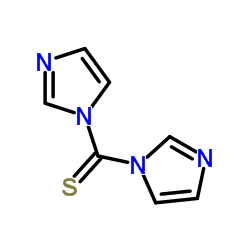 CAS#:6160-65-2
CAS#:6160-65-2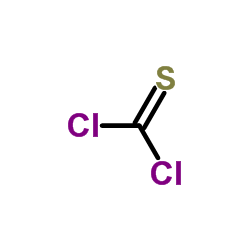 CAS#:463-71-8
CAS#:463-71-8 CAS#:19457-13-7
CAS#:19457-13-7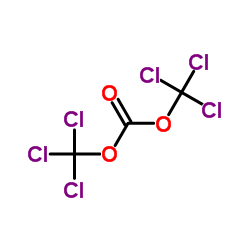 CAS#:32315-10-9
CAS#:32315-10-9 CAS#:18085-24-0
CAS#:18085-24-0 CAS#:35653-54-4
CAS#:35653-54-4 CAS#:6815-00-5
CAS#:6815-00-5 CAS#:66491-03-0
CAS#:66491-03-0 CAS#:1196-38-9
CAS#:1196-38-9 CAS#:21198-23-2
CAS#:21198-23-2 CAS#:15093-42-2
CAS#:15093-42-2 CAS#:22245-96-1
CAS#:22245-96-1![1-[(3-methyl-4-phenyl-pyridin-2-yl)methylideneamino]-3-phenethyl-thiourea structure](https://image.chemsrc.com/caspic/211/76609-57-9.png) CAS#:76609-57-9
CAS#:76609-57-9 CAS#:6552-60-9
CAS#:6552-60-9 CAS#:66491-04-1
CAS#:66491-04-1
