1-Methoxynapthalene
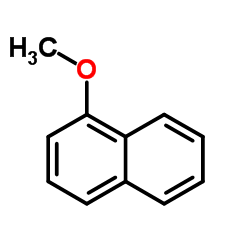
1-Methoxynapthalene structure
|
Common Name | 1-Methoxynapthalene | ||
|---|---|---|---|---|
| CAS Number | 2216-69-5 | Molecular Weight | 158.197 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 268.3±9.0 °C at 760 mmHg | |
| Molecular Formula | C11H10O | Melting Point | 5 °C | |
| MSDS | Chinese USA | Flash Point | 102.3±8.0 °C | |
Use of 1-Methoxynapthalene1-Methoxynaphthalene is used as the substrate to investigate the activity of cytochrome c peroxidase (CcP). 1-Methoxynaphthalene also can be used to synthesize prenyl naphthalen-ols[1][2]. |
| Name | 1-Methoxynaphthalene |
|---|---|
| Synonym | More Synonyms |
| Description | 1-Methoxynaphthalene is used as the substrate to investigate the activity of cytochrome c peroxidase (CcP). 1-Methoxynaphthalene also can be used to synthesize prenyl naphthalen-ols[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 268.3±9.0 °C at 760 mmHg |
| Melting Point | 5 °C |
| Molecular Formula | C11H10O |
| Molecular Weight | 158.197 |
| Flash Point | 102.3±8.0 °C |
| Exact Mass | 158.073166 |
| PSA | 9.23000 |
| LogP | 3.36 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.604 |
| Water Solubility | immiscible |
| Personal Protective Equipment | Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi |
| Safety Phrases | S23-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | QJ9465500 |
| HS Code | 29093090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2909309090 |
|---|---|
| Summary | 2909309090 other aromatic ethers and their halogenated, sulphonated, nitrated or nitrosated derivatives VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
Production of novel antioxidative prenyl naphthalen-ols by combinational bioconversion with dioxygenase PhnA1A2A3A4 and prenyltransferase NphB or SCO7190.
Biosci. Biotechnol. Biochem. 75(3) , 505-10, (2011) We performed combinational bioconversion of substituted naphthalenes with PhnA1A2A3A4 (an aromatic dihydroxylating dioxygenase from marine bacterium Cycloclasticus sp. strain A5) and prenyltransferase... |
|
|
Peroxygenase activity of cytochrome c peroxidase and three apolar distal heme pocket mutants: hydroxylation of 1-methoxynaphthalene.
BMC Biochem. 14(1) , 19, (2013) The cytochrome P450s are monooxygenases that insert oxygen functionalities into a wide variety of organic substrates with high selectivity. There is interest in developing efficient catalysts based on... |
| Naphthalene, 1-methoxy- |
| 1-naphthol methyl ether |
| Naphthalene,1-methoxy |
| EINECS 218-696-1 |
| MFCD00003924 |
| 1-Methoxynapthalene |
| methoxy-naphthalene |
| 1-naphthyl methyl ether |
| Methyl 1-naphthyl ether |
| α-Methoxynaphthalene |
| methyl naphthalen-1-yl ether |
| methyl naphthyl ether |
| 1-methoxy naphthalene |
| 1-Methoxynaphthalene |
 CAS#:90-15-3
CAS#:90-15-3 CAS#:77-78-1
CAS#:77-78-1 CAS#:112183-02-5
CAS#:112183-02-5 CAS#:5961-55-7
CAS#:5961-55-7 CAS#:67-56-1
CAS#:67-56-1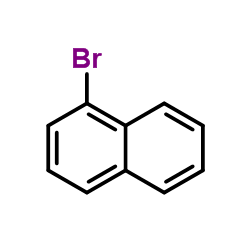 CAS#:90-11-9
CAS#:90-11-9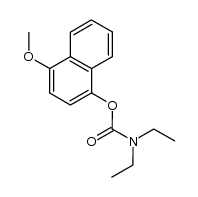 CAS#:1201594-02-6
CAS#:1201594-02-6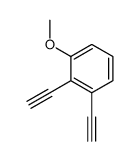 CAS#:412041-47-5
CAS#:412041-47-5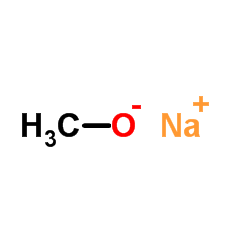 CAS#:124-41-4
CAS#:124-41-4 CAS#:5467-58-3
CAS#:5467-58-3 CAS#:10465-20-0
CAS#:10465-20-0 CAS#:119-64-2
CAS#:119-64-2 CAS#:1008-19-1
CAS#:1008-19-1 CAS#:66324-83-2
CAS#:66324-83-2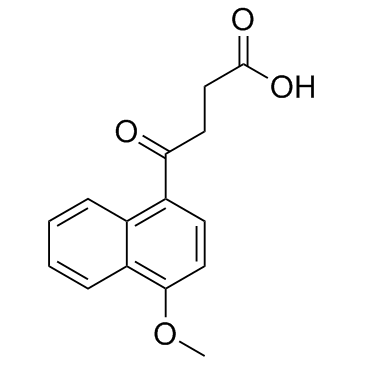 CAS#:3562-99-0
CAS#:3562-99-0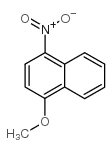 CAS#:4900-63-4
CAS#:4900-63-4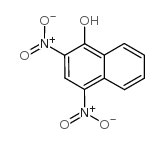 CAS#:605-69-6
CAS#:605-69-6 CAS#:4900-62-3
CAS#:4900-62-3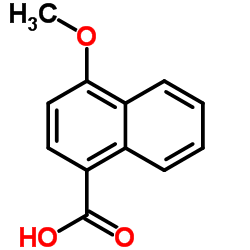 CAS#:13041-62-8
CAS#:13041-62-8
