Caerulomycin A
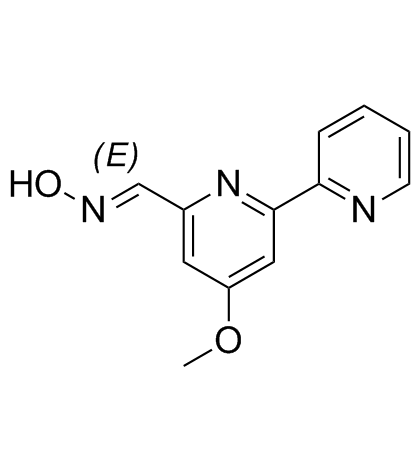
Caerulomycin A structure
|
Common Name | Caerulomycin A | ||
|---|---|---|---|---|
| CAS Number | 21802-37-9 | Molecular Weight | 229.23500 | |
| Density | 1.23g/cm3 | Boiling Point | 400.4ºC at 760mmHg | |
| Molecular Formula | C12H11N3O2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 195.9ºC | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of Caerulomycin ACaerulomycin A (Cerulomycin; Caerulomycin), an antifungal compound, induces generation of T cells, enhances TGF-β-Smad3 protein signaling via suppressing interferon-γ-induced STAT1 signaling. Antifungal and antibiotic activity, and used in autoimmune diseases[1]. |
| Name | caerulomycin A |
|---|---|
| Synonym | More Synonyms |
| Description | Caerulomycin A (Cerulomycin; Caerulomycin), an antifungal compound, induces generation of T cells, enhances TGF-β-Smad3 protein signaling via suppressing interferon-γ-induced STAT1 signaling. Antifungal and antibiotic activity, and used in autoimmune diseases[1]. |
|---|---|
| Related Catalog | |
| Target |
Antifungal[1] TGF-β-Smad3[1] |
| References |
| Density | 1.23g/cm3 |
|---|---|
| Boiling Point | 400.4ºC at 760mmHg |
| Molecular Formula | C12H11N3O2 |
| Molecular Weight | 229.23500 |
| Flash Point | 195.9ºC |
| Exact Mass | 229.08500 |
| PSA | 67.60000 |
| LogP | 1.96030 |
| Storage condition | 2-8℃ |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H315-H319 |
| Precautionary Statements | P301 + P310-P305 + P351 + P338 |
| RIDADR | UN 2811 6.1 / PGIII |
| HS Code | 2933399090 |
| Precursor 8 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
[Development of a genetic modification system for caerulomycin producer Actinoalloteichus sp. WH1-2216-6].
Wei Sheng Wu Xue Bao 51(8) , 1032-41, (2011) In order to enable the caerulomyicn biosynthetic study by in vivo gene disruptions, it is crucial to develop a genetic modification system for the producer Actinoalloteichus sp. WH1-2216-6.The spore g... |
|
|
Total synthesis of caerulomycin C via the halogen dance reaction.
Org. Lett. 4(14) , 2385-8, (2002) [reaction: see text] The total synthesis of caerulomycin C is described. Key steps in this synthesis utilize 1,2-, 1,3-, and 1,4-halogen dance reactions for the functionalization of the pyridine ring. |
|
|
Caerulomycin, an antifungal antibiotic with marked in vitro and in vivo activity against Entamoeba histolytica.
Z. Parasitenkd. 70(5) , 569-73, (1984) The anti-amoebic action of the bipyridyl antibiotic caerulomycin was assessed in vitro and in vivo using various strains of Entamoeba histolytica from polyxenic, axenic and monoxenic cultures. Minimum... |
| CERULOMYCIN |
| (E)-4-methoxy-2,2'-bipyridyl-6-aldoxime |
| CAERULOMYCIN A |
| 4-Methoxy-2,2'-bipyridine-6-carbaldehyde (E)-oxime |
| 4-Methoxy-[2,2']bipyridyl-6-carbaldehyd-(E)-oxim |
| Caerulomycin,Cerulomycin |
| (2,2'-Bipyridine)-6-carboxaldehyde,4-methoxy-,oxime,(E) |
| (E)-4-Methoxy-[2,2'-bipyridine]-6-carbaldehyde oxime |
| 4-methoxy-[2,2']bipyridinyl-6-carbaldehyde oxime |
| 4-methoxy-[2,2']bipyridyl-6-carbaldehyde-(E)-oxime |
| Caerulomycin A |
| CAERULOMYCIN |
 CAS#:366-18-7
CAS#:366-18-7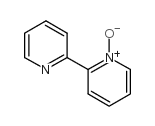 CAS#:33421-43-1
CAS#:33421-43-1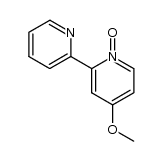 CAS#:14163-05-4
CAS#:14163-05-4 CAS#:14163-00-9
CAS#:14163-00-9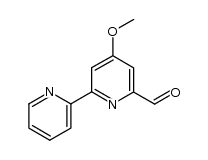 CAS#:114115-50-3
CAS#:114115-50-3 CAS#:205052-95-5
CAS#:205052-95-5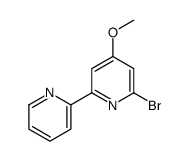 CAS#:205052-93-3
CAS#:205052-93-3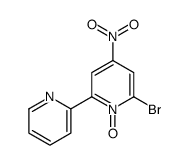 CAS#:205052-97-7
CAS#:205052-97-7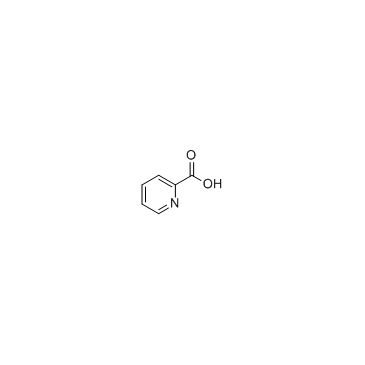 CAS#:98-98-6
CAS#:98-98-6