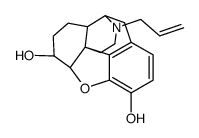
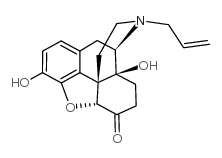
6-Alpha Naloxol
6-Alpha Naloxol structure
|
Common Name | 6-Alpha Naloxol | ||
|---|---|---|---|---|
| CAS Number | 20410-95-1 | Molecular Weight | 329.39000 | |
| Density | 1.43g/cm3 | Boiling Point | 532.5ºC at 760mmHg | |
| Molecular Formula | C19H23NO4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 275.8ºC | |
Use of 6-Alpha Naloxol6-Alpha Naloxol(Alpha-Naloxol) is an opioid antagonist closely related to naloxone; a human metabolite of naloxone.IC50 value:Target: opioid antagonistWhen responding over the entire 30 min operant session was examined, naloxone was only 5-fold more potent than 6-alpha-naloxol in suppressing operant responding under Morphine Na ve conditions, but this increased to a 65-fold potency difference after Single or Repeat Morphine pretreatment. Examination of the relative potency of these antagonists in the Early Phase of operant testing (5-15 min post-antagonist) revealed an even greater 100-fold potency difference between naloxone and 6-alpha-naloxol, but in the Late Phase of testing (25-35 min post-antagonist), this had declined to a 9-fold potency difference, comparable to the relative potency of naloxone to 6-alpha-naloxol under Morphine-Na ve conditions. |
| Name | 6 α-naloxol |
|---|---|
| Synonym | More Synonyms |
| Description | 6-Alpha Naloxol(Alpha-Naloxol) is an opioid antagonist closely related to naloxone; a human metabolite of naloxone.IC50 value:Target: opioid antagonistWhen responding over the entire 30 min operant session was examined, naloxone was only 5-fold more potent than 6-alpha-naloxol in suppressing operant responding under Morphine Na ve conditions, but this increased to a 65-fold potency difference after Single or Repeat Morphine pretreatment. Examination of the relative potency of these antagonists in the Early Phase of operant testing (5-15 min post-antagonist) revealed an even greater 100-fold potency difference between naloxone and 6-alpha-naloxol, but in the Late Phase of testing (25-35 min post-antagonist), this had declined to a 9-fold potency difference, comparable to the relative potency of naloxone to 6-alpha-naloxol under Morphine-Na ve conditions. |
|---|---|
| Related Catalog | |
| References |
[1]. Weinstein SH, et al. Metabolites of naloxone in human urine. J Pharm Sci. 1971 Oct;60(10):1567-8. |
| Density | 1.43g/cm3 |
|---|---|
| Boiling Point | 532.5ºC at 760mmHg |
| Molecular Formula | C19H23NO4 |
| Molecular Weight | 329.39000 |
| Flash Point | 275.8ºC |
| Exact Mass | 329.16300 |
| PSA | 73.16000 |
| LogP | 1.03110 |
| Vapour Pressure | 3.62E-12mmHg at 25°C |
| Index of Refraction | 1.701 |
| InChIKey | HMWHERQFMBEHNG-AQQQZIQISA-N |
| SMILES | C=CCN1CCC23c4c5ccc(O)c4OC2C(O)CCC3(O)C1C5 |
|
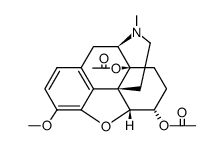
~93% 
6-Alpha Naloxol CAS#:20410-95-1 |
| Literature: MALLINCKRODT INC. Patent: WO2008/137672 A1, 2008 ; Location in patent: Page/Page column 25 ; |
|
~% 
6-Alpha Naloxol CAS#:20410-95-1 |
| Literature: Rezaie, Robert; Bailey, Timothy S. Patent: US2010/36128 A1, 2010 ; Location in patent: Page/Page column 7 ; |
|
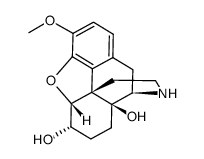
~63% 
6-Alpha Naloxol CAS#:20410-95-1 |
| Literature: Hosztafi, Sandor; Berenyi, Sandor; Toth, Geza; Makleit, Sandor Monatshefte fuer Chemie, 1992 , vol. 123, # 5 p. 435 - 442 |
|
~% 
6-Alpha Naloxol CAS#:20410-95-1 |
| Literature: Hahn; Fishman Journal of Organic Chemistry, 1975 , vol. 40, # 1 p. 31 - 34 |
|
~% 
6-Alpha Naloxol CAS#:20410-95-1 |
| Literature: Hosztafi, Sandor; Berenyi, Sandor; Toth, Geza; Makleit, Sandor Monatshefte fuer Chemie, 1992 , vol. 123, # 5 p. 435 - 442 |
|
~% 
6-Alpha Naloxol CAS#:20410-95-1 |
| Literature: Hosztafi, Sandor; Berenyi, Sandor; Toth, Geza; Makleit, Sandor Monatshefte fuer Chemie, 1992 , vol. 123, # 5 p. 435 - 442 |
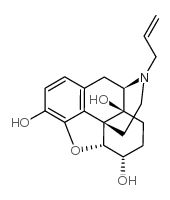
| 6a-Naloxol |
| 6-Alpha Naloxol |
![(4R,4aS,7S,7aR,12bS)-3-allyl-9-methoxy-2,3,4,4a,5,6,7,7a-octahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-4a,7-diol structure](https://image.chemsrc.com/caspic/345/116388-84-2.png)