Clofazimine
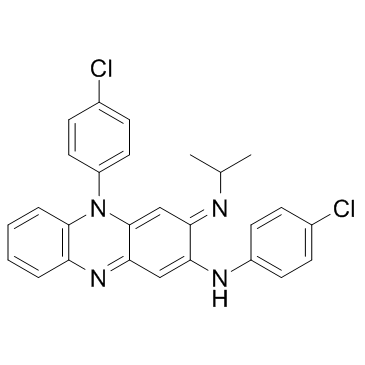
Clofazimine structure
|
Common Name | Clofazimine | ||
|---|---|---|---|---|
| CAS Number | 2030-63-9 | Molecular Weight | 473.396 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 566.9±50.0 °C at 760 mmHg | |
| Molecular Formula | C27H22Cl2N4 | Melting Point | 210-212° | |
| MSDS | Chinese USA | Flash Point | 296.7±30.1 °C | |
Use of ClofazimineClofazimine is a fat-soluble iminophenazine dye, has a marked anti-inflammatory effect, has been used in combination with other antimycobacterial drugs to treat AIDS and Crohn's disease. |
| Name | clofazimine |
|---|---|
| Synonym | More Synonyms |
| Description | Clofazimine is a fat-soluble iminophenazine dye, has a marked anti-inflammatory effect, has been used in combination with other antimycobacterial drugs to treat AIDS and Crohn's disease. |
|---|---|
| Related Catalog |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 566.9±50.0 °C at 760 mmHg |
| Melting Point | 210-212° |
| Molecular Formula | C27H22Cl2N4 |
| Molecular Weight | 473.396 |
| Flash Point | 296.7±30.1 °C |
| Exact Mass | 472.122162 |
| PSA | 42.21000 |
| LogP | 7.26 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.667 |
| Water Solubility | Practically insoluble in water, soluble in methylene chloride, very slightly soluble in ethanol (96 per cent). It shows polymorphism (5.9). |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | SG1578000 |
| HS Code | 35040000 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
The efflux pump inhibitor timcodar improves the potency of antimycobacterial agents.
Antimicrob. Agents Chemother. 59(3) , 1534-41, (2015) Previous studies indicated that inhibition of efflux pumps augments tuberculosis therapy. In this study, we used timcodar (formerly VX-853) to determine if this efflux pump inhibitor could increase th... |
|
|
Clofazimine shortens the duration of the first-line treatment regimen for experimental chemotherapy of tuberculosis.
Proc. Natl. Acad. Sci. U. S. A. 112(3) , 869-74, (2015) A key drug for the treatment of leprosy, clofazimine has recently been associated with highly effective and significantly shortened regimens for the treatment of multidrug-resistant tuberculosis (TB).... |
|
|
Anticancer efficacy and toxicokinetics of a novel paclitaxel-clofazimine nanoparticulate co-formulation.
Drug Deliv Transl Res 5 , 257-67, (2015) Contemporary chemotherapy is limited by disseminated, resistant cancer. Targeting nanoparticulate drug delivery systems that encapsulate synergistic drug combinations are a rational means to increase ... |
| 2-Phenazinamine, N,5-bis(4-chlorophenyl)-3,5-dihydro-3-[(1-methylethyl)imino]- |
| N,5-Bis(4-chlorophenyl)-3-(isopropylimino)-3,5-dihydro-2-phenazinamine |
| Clofaziminum [INN-Latin] |
| 2-Phenazinamine, N,5-bis(4-chlorophenyl)-3,5-dihydro-3-((1-methylethyl)imino)- |
| Clofazimine |
| Clofaziminum |
| N,5-bis(4-chlorophenyl)-3-propan-2-yliminophenazin-2-amine |
| Chlofazimine |
| Clofazimine (N,5-Bis(4-chlorophenyl)-3,5-dihydro-3-(isopropylimino)phenazin-2-amine |
| Clofazimina [INN-Spanish] |
| Lamprene |
| Lampren |
| 2-(4-chloro-anilino)-5-(4-chloro-phenyl)-3-isopropylamino-phenazinium-betaine |
| 2-(4-Chlor-anilino)-5-(4-chlor-phenyl)-3-isopropylamino-phenazinium-betain |
| B 663 (Pharmaceutical) |
| N,5-Bis(4-chlorophenyl)-3,5-dihydro-3-(isopropylimino)phenazin-2-amine |
| Clofazimina |
![1,5-bis(4-chlorophenyl)-2,2-dimethylimidazo[4,5-b]phenazine Structure](https://image.chemsrc.com/caspic/115/84803-71-4.png) CAS#:84803-71-4
CAS#:84803-71-4
