Prazosin hydrochloride

Prazosin hydrochloride structure
|
Common Name | Prazosin hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 19237-84-4 | Molecular Weight | 419.862 | |
| Density | N/A | Boiling Point | 638.4ºC at 760 mmHg | |
| Molecular Formula | C19H22ClN5O4 | Melting Point | 277 - 280 °C | |
| MSDS | Chinese USA | Flash Point | 339.9ºC | |
| Symbol |


GHS07, GHS08 |
Signal Word | Warning | |
Use of Prazosin hydrochloridePrazosin is an alpha-adrenergic blocker and is a sympatholytic drug used to treat high blood pressure and anxiety, PTSD, and panic disorder.Target: Adrenergic ReceptorPrazosin, is a sympatholytic drug used to treat high blood pressure and anxiety, PTSD, andpanic disorder. It is an alpha-adrenergic blocker that is specific for the alpha-1 receptors. These receptors are found on vascular smooth muscle, where they are responsible for the vasoconstrictive action of norepinephrine. They are also found throughout the central nervous system. As of 2013, prazosin is off-patent in the US, and the FDA has approved at least one generic manufacturer.In addition to its alpha-blocking activity, prazosin is an antagonist of the MT3 receptor (which is not present in humans), with selectivity for this receptor over the MT1 and MT2 receptors.Prazosin is orally active and has a minimal effect on cardiac function due to its alpha-1 receptor selectivity. However, when prazosin is initially started, heart rate and contractility go up in order to maintain the pre-treatment blood pressures because the body has reached homeostasis at its abnormally high blood pressure. The blood pressure lowering effect becomes apparent when prazosin is taken for longer periods of time. The heart rate and contractility go back down over time and blood pressure decreases. |
| Name | prazosin hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Prazosin is an alpha-adrenergic blocker and is a sympatholytic drug used to treat high blood pressure and anxiety, PTSD, and panic disorder.Target: Adrenergic ReceptorPrazosin, is a sympatholytic drug used to treat high blood pressure and anxiety, PTSD, andpanic disorder. It is an alpha-adrenergic blocker that is specific for the alpha-1 receptors. These receptors are found on vascular smooth muscle, where they are responsible for the vasoconstrictive action of norepinephrine. They are also found throughout the central nervous system. As of 2013, prazosin is off-patent in the US, and the FDA has approved at least one generic manufacturer.In addition to its alpha-blocking activity, prazosin is an antagonist of the MT3 receptor (which is not present in humans), with selectivity for this receptor over the MT1 and MT2 receptors.Prazosin is orally active and has a minimal effect on cardiac function due to its alpha-1 receptor selectivity. However, when prazosin is initially started, heart rate and contractility go up in order to maintain the pre-treatment blood pressures because the body has reached homeostasis at its abnormally high blood pressure. The blood pressure lowering effect becomes apparent when prazosin is taken for longer periods of time. The heart rate and contractility go back down over time and blood pressure decreases. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 638.4ºC at 760 mmHg |
|---|---|
| Melting Point | 277 - 280 °C |
| Molecular Formula | C19H22ClN5O4 |
| Molecular Weight | 419.862 |
| Flash Point | 339.9ºC |
| Exact Mass | 419.136017 |
| PSA | 107.68000 |
| LogP | 2.51960 |
| Vapour Pressure | 3.4E-16mmHg at 25°C |
| InChIKey | WFXFYZULCQKPIP-UHFFFAOYSA-N |
| SMILES | COc1cc2nc(N3CCN(C(=O)c4ccco4)CC3)nc(N)c2cc1OC.Cl |
| Storage condition | Store at RT |
| Water Solubility | H2O: 1 mg/mL, clear, colorless |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319-H335-H361 |
| Precautionary Statements | P280-P301 + P312 + P330-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22;R36/37/38;R62 |
| Safety Phrases | S28-S36 |
| RIDADR | 3249 |
| WGK Germany | 3 |
| RTECS | VA1350000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
|
~83% 
Prazosin hydroc... CAS#:19237-84-4 |
| Literature: Journal of Heterocyclic Chemistry, , vol. 17, # 4 p. 797 - 798 |
|
~% 
Prazosin hydroc... CAS#:19237-84-4 |
| Literature: US4092315 A1, ; |
|
~% 
Prazosin hydroc... CAS#:19237-84-4 |
| Literature: Journal of Heterocyclic Chemistry, , vol. 17, # 4 p. 797 - 798 |
|
~% 
Prazosin hydroc... CAS#:19237-84-4 |
| Literature: Journal of Heterocyclic Chemistry, , vol. 17, # 4 p. 797 - 798 |
|
~% 
Prazosin hydroc... CAS#:19237-84-4 |
| Literature: Journal of Heterocyclic Chemistry, , vol. 17, # 4 p. 797 - 798 |
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain canc... |
|
|
Different compartmentation of responses to brain natriuretic peptide and C-type natriuretic peptide in failing rat ventricle.
J. Pharmacol. Exp. Ther. 350(3) , 681-90, (2014) We previously found a negative inotropic (NIR) and positive lusitropic response (LR) to C-type natriuretic peptide (CNP) in the failing heart ventricle. In this study, we investigated and compared the... |
|
|
Genetic mapping of targets mediating differential chemical phenotypes in Plasmodium falciparum.
Nat. Chem. Biol. 5 , 765-71, (2009) Studies of gene function and molecular mechanisms in Plasmodium falciparum are hampered by difficulties in characterizing and measuring phenotypic differences between individual parasites. We screened... |
| 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl)-piperazine * HCl |
| Furazosin hydrochloride |
| 2-[4-(furan-2-ylcarbonyl)pipérazin-1-yl]-6,7-diméthoxyquinazolin-4-amine chlorhydrate |
| Methanone, [4-(4-amino-6,7-dimethoxy-2-quinazolinyl)-1-piperazinyl]-2-furanyl-, hydrochloride (1:1) |
| 2-[4-(furan-2-ylcarbonyl)piperazin-1-yl]-6,7-dimethoxyquinazolin-4-amine hydrochloride |
| Vasoflex |
| Pratsiol |
| [4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl]-(furan-2-yl)methanone,hydrochloride |
| [4-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-1-piperazinyl](2-furyl)methanone hydrochloride |
| prazosine hydrochloride |
| 2-[4-(Furan-2-ylcarbonyl)piperazin-1-yl]-6,7-dimethoxychinazolin-4-aminhydrochlorid |
| [4-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-1-piperazinyl](2-furyl)methanone hydrochloride (1:1) |
| EINECS 242-903-4 |
| 4-Amino-6,7-dimethoxy-2-[4-(2-furoyl)piperazine-1-yl]quinazoline hydrochloride |
| 1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl)piperazine Hydrochloride |
| Deprazolin |
| [4-(4-Amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl](2-furyl)methanone hydrochloride (1:1) |
| [4-(4-Amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl](furan-2-yl)methanone hydrochloride |
| Prazosin hydrochloride |
| 6,7-dimethoxy-4-amino-2-[4-(2-furoyl)-1-piperazinyl]quinazoline hydrochloride |
| Hypovasole |
| Prazosin HCl |
| Hypovase |
| MFCD00058177 |
| [4-(4-Amino-6,7-dimethoxychinazolin-2-yl)piperazin-1-yl](furan-2-yl)methanonhydrochlorid |
| Peripress |
| Prazosin (hydrochloride) |
![Methyl N-(3,4-Dimethoxy-6-cyanophenyl)-[4-(2-furoyl)piperazin-1-yl]thioformamidate structure](https://image.chemsrc.com/caspic/427/67817-59-8.png)
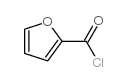
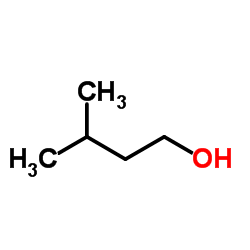

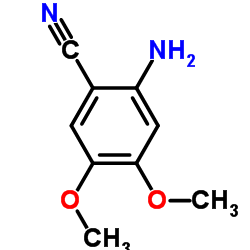
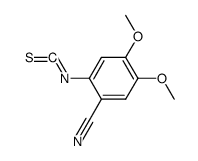
 CAS#:63590-64-7
CAS#:63590-64-7
