L-Cysteine methyl ester hydrochloride
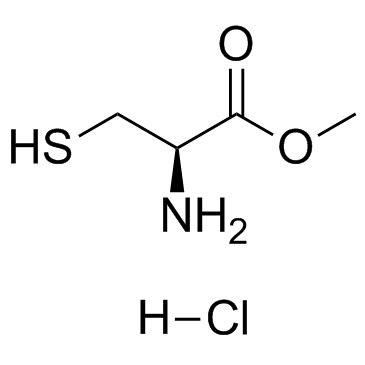
L-Cysteine methyl ester hydrochloride structure
|
Common Name | L-Cysteine methyl ester hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 18598-63-5 | Molecular Weight | 171.646 | |
| Density | N/A | Boiling Point | 197.2ºC at 760 mmHg | |
| Molecular Formula | C4H10ClNO2S | Melting Point | 142 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 73.1ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of L-Cysteine methyl ester hydrochlorideMecysteine hydrochloride is an antitussive, and an expectorant agent, used to relieve breathing difficulties caused by mucus. |
| Name | methyl (2R)-2-amino-3-sulfanylpropanoate,hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Mecysteine hydrochloride is an antitussive, and an expectorant agent, used to relieve breathing difficulties caused by mucus. |
|---|---|
| Related Catalog |
| Boiling Point | 197.2ºC at 760 mmHg |
|---|---|
| Melting Point | 142 °C (dec.)(lit.) |
| Molecular Formula | C4H10ClNO2S |
| Molecular Weight | 171.646 |
| Flash Point | 73.1ºC |
| Exact Mass | 171.012070 |
| PSA | 91.12000 |
| LogP | 0.91880 |
| Vapour Pressure | 0.384mmHg at 25°C |
| Index of Refraction | -2.5 ° (C=20, MeOH) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | HA2460000 |
| HS Code | 2930909090 |
| Precursor 0 | |
|---|---|
| DownStream 9 | |
| HS Code | 2930909090 |
|---|---|
| Summary | 2930909090. other organo-sulphur compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Steroidal sapogenins and glycosides from the fibrous roots of Ophiopogon japonicus and Liriope spicata var. prolifera with anti-inflammatory activity.
Chem. Pharm. Bull. 63(3) , 187-94, (2015) Two new steroidal glycosides (1 and 2), together with 15 known compounds (3-17) were isolated from the fibrous roots of Ophiopogon japonicus, and three new steroidal glycosides (18-20), together with ... |
|
|
Triterpene glycosides and other polar constituents of shea (Vitellaria paradoxa) kernels and their bioactivities.
Phytochemistry 108 , 157-70, (2014) The MeOH extract of defatted shea (Vitellaria paradoxa; Sapotaceae) kernels was investigated for its constituents, and fifteen oleanane-type triterpene acids and glycosides, two steroid glucosides, tw... |
|
|
The role of hydrogen bonding in the selectivity of L-cysteine methyl ester (CYSM) and L-cysteine ethyl ester (CYSE) for chloride ion.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 61(5) , 845-54, (2005) The interaction of cysteamine (CY), L-cysteine methyl ester (CYSM), and L-cysteine ethyl ester (CYSE) with nitrate, sulfate, perchlorate, dihydrogen phosphate, and chloride ions was investigated using... |
| Actiol |
| H-Cys-OMe·HCl |
| Mecystein HCl |
| cysteine methyl ester hydrochloride |
| methyl L-cysteinate hydrochloride |
| Acdrile |
| MFCD00038985 |
| Methyl L-cysteinate hydrochloride (1:1) |
| EINECS 242-435-0 |
| L-Cysteine, methyl ester, hydrochloride (1:1) |
| hydrochloride*H-Cys-OMe |
| Methyl cysteine HCl |
| H-Cys-OMe.HCl |
| L-Cysteine Methyl Ester Hydrochloride |
| L-Cysteinemethylesterhydrochloride |
 CAS#:1069-29-0
CAS#:1069-29-0 CAS#:3532-25-0
CAS#:3532-25-0 CAS#:32854-09-4
CAS#:32854-09-4 CAS#:65-85-0
CAS#:65-85-0 CAS#:92814-42-1
CAS#:92814-42-1 CAS#:19547-88-7
CAS#:19547-88-7 CAS#:251294-64-1
CAS#:251294-64-1 CAS#:127761-77-7
CAS#:127761-77-7 CAS#:56-89-3
CAS#:56-89-3
