Contezolid acefosamil sodium
Modify Date: 2024-01-12 15:43:21
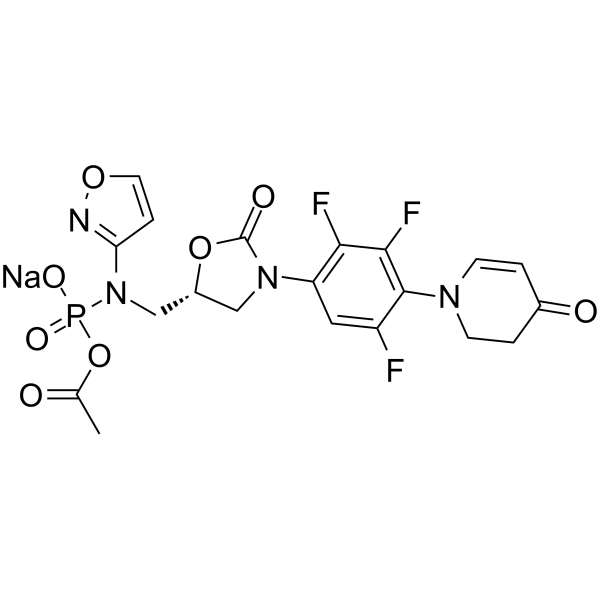
Contezolid acefosamil sodium structure
|
Common Name | Contezolid acefosamil sodium | ||
|---|---|---|---|---|
| CAS Number | 1807365-35-0 | Molecular Weight | 552.33 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C20H17F3N4NaO8P | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Contezolid acefosamil sodiumContezolid acefosamil sodium (MRX-4), a new and orally active oxazolidinone, is an antibiotic in study for complicated skin and soft tissue infections (cSSTI) caused by resistant Gram-positive bacteria. Contezolid acefosamil sodium (MRX-4) markedly reduces potential for myelosuppression and monoamine oxidase inhibition (MAOI)[1][2]. |
| Name | Contezolid acefosamil |
|---|---|
| Synonym | More Synonyms |
| Description | Contezolid acefosamil sodium (MRX-4), a new and orally active oxazolidinone, is an antibiotic in study for complicated skin and soft tissue infections (cSSTI) caused by resistant Gram-positive bacteria. Contezolid acefosamil sodium (MRX-4) markedly reduces potential for myelosuppression and monoamine oxidase inhibition (MAOI)[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Contezolid (MRX-I) is highly potent against all Grampositive clinical isolates of staphylococci, streptococci, and enterococci, including MDR organisms such as MRSA, methicilline-resistant Streptococcus epidermidis (MRSE), penicillin-resistant Streptococci (PRSP), and VRE[2]. |
| In Vivo | Oral absorption of Contezolid (MRX-I) occurrs rapidly in mouse, rat, and dog, with peak plasma concentrations observed at 0.5−2.6 h postdose. In mouse, rat, and dog, respectively, PK parameters are determined as follows: dose-normalized Cmax/dose was 524, 1065, and 259 ng/mL/(mg/kg); dose-normalized AUC0−t/dose was 1654, 3703, and 1664 ng•h/mL/(mg/kg); T1/2 is 1, 1.5, and 3 h; and the oral bioavailability is 69%, 109%, and 37%[2]. Contezolid (MRX-I) exhibits no obvious toxicity[2]. Contezolid (MRX-I, 100 mg/kg, once daily) significantly reduced the bacterial load in lungs compared to the untreated early and late controls[3]. Animal Model: BALB/c mice infected intranasally with M. tuberculosis Erdman[3]. Dosage: 100, 50 (twice), 25 (twice) mg/kg. Administration: Gavage, once or twice daily, five days per week for four weeks. Result: Significantly reduced the CFU recovered from the lungs compared to the early and late control mice (P < 0.05). Twice daily MRX-I at 50mg/kg and 25 mg/kg were significantly better than the late control mice (P < 0.05). Once daily MRX-I at 100 mg/kg was significantly better than twice daily 50 mg/kg and 25 mg/kg (P < 0.05). There was no statistical difference between twice daily 50 mg/kg of MRX-I and 25mg/kg (P > 0.05). Animal Model: Rats[2]. Dosage: 20, 100, and 200/300 mg/kg/day. Administration: Orally twice daily. Result: No mortality was observed. |
| References |
| Molecular Formula | C20H17F3N4NaO8P |
|---|---|
| Molecular Weight | 552.33 |
| Exact Mass | 552.063354 |
| Phosphoramidic acid, N-[[(5R)-3-[4-(3,4-dihydro-4-oxo-1(2H)-pyridinyl)-2,3,5-trifluorophenyl]-2-oxo-5-oxazolidinyl]methyl]-N-3-isoxazolyl-, acetyl ester, sodium salt (1:1) |
| T79C086548 |
| UNII:T79C086548 |
| UNII-T79C086548 |
| Contezolid acefosamil |