macrocarpal J
Modify Date: 2025-08-25 14:59:19
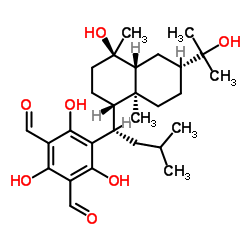
macrocarpal J structure
|
Common Name | macrocarpal J | ||
|---|---|---|---|---|
| CAS Number | 179603-47-5 | Molecular Weight | 490.629 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 569.5±50.0 °C at 760 mmHg | |
| Molecular Formula | C28H42O7 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 312.2±26.6 °C | |
Use of macrocarpal JMacrocarpal J, a phloroglucinol?sesquiterpene-coupled compound, can be isolated from the leaves of Eucalyptus globulus[1]. |
| Name | 2,4,6-trihydroxy-5-[(1S)-1-[(4S)-4-hydroxy-6-(2-hydroxypropan-2-yl)-4,8a-dimethyl-1,2,3,4a,5,6,7,8-octahydronaphthalen-1-yl]-3-methylbutyl]benzene-1,3-dicarbaldehyde |
|---|---|
| Synonym | More Synonyms |
| Description | Macrocarpal J, a phloroglucinol?sesquiterpene-coupled compound, can be isolated from the leaves of Eucalyptus globulus[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 569.5±50.0 °C at 760 mmHg |
| Molecular Formula | C28H42O7 |
| Molecular Weight | 490.629 |
| Flash Point | 312.2±26.6 °C |
| Exact Mass | 490.293060 |
| PSA | 135.29000 |
| LogP | 7.49 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.600 |
| Hazard Codes | Xi |
|---|
| 1,3-Benzenedicarboxaldehyde, 5-[(1S)-1-[(1S,4R,4aR,6R,8aS)-decahydro-4-hydroxy-6-(1-hydroxy-1-methylethyl)-4,8a-dimethyl-1-naphthalenyl]-3-methylbutyl]-2,4,6-trihydroxy- |
| 2,4,6-Trihydroxy-5-{(1S)-1-[(1S,4R,4aR,6R,8aS)-4-hydroxy-6-(2-hydroxy-2-propanyl)-4,8a-dimethyldecahydro-1-naphthalenyl]-3-methylbutyl}isophthalaldehyde |
| Macrocarpal J |

