Boc-His-OH
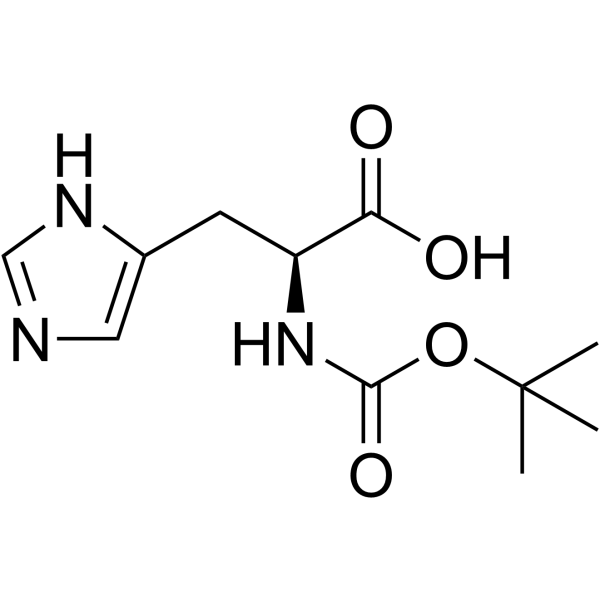
Boc-His-OH structure
|
Common Name | Boc-His-OH | ||
|---|---|---|---|---|
| CAS Number | 17791-52-5 | Molecular Weight | 255.270 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 522.2±45.0 °C at 760 mmHg | |
| Molecular Formula | C11H17N3O4 | Melting Point | 195 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 269.6±28.7 °C | |
Use of Boc-His-OHN-tert-Butyloxycarbonyl-L-histidine is a histidine derivative[1]. |
| Name | (2S)-3-(1H-imidazol-5-yl)-2-[(2-methylpropan-2-yl)oxycarbonylamino]propanoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | N-tert-Butyloxycarbonyl-L-histidine is a histidine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 522.2±45.0 °C at 760 mmHg |
| Melting Point | 195 °C (dec.)(lit.) |
| Molecular Formula | C11H17N3O4 |
| Molecular Weight | 255.270 |
| Flash Point | 269.6±28.7 °C |
| Exact Mass | 255.121902 |
| PSA | 104.31000 |
| LogP | 0.58 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.542 |
| InChIKey | AYMLQYFMYHISQO-QMMMGPOBSA-N |
| SMILES | CC(C)(C)OC(=O)NC(Cc1cnc[nH]1)C(=O)O |
|
~91% 
Boc-His-OH CAS#:17791-52-5 |
| Literature: Tetrahedron Letters, , vol. 43, # 2 p. 311 - 313 |
|
~88% 
Boc-His-OH CAS#:17791-52-5 |
| Literature: Tetrahedron Letters, , vol. 43, # 2 p. 311 - 313 |
|
~% 
Boc-His-OH CAS#:17791-52-5 |
| Literature: Chemistry of Natural Compounds, , vol. 18, # 3 p. 322 - 327 Khimiya Prirodnykh Soedinenii, , # 3 p. 349 - 354 |
|
~% 
Boc-His-OH CAS#:17791-52-5 |
| Literature: Chemistry of Natural Compounds, , vol. 18, # 3 p. 322 - 327 Khimiya Prirodnykh Soedinenii, , # 3 p. 349 - 354 |
|
~% 
Boc-His-OH CAS#:17791-52-5 |
| Literature: Chemical & Pharmaceutical Bulletin, , vol. 30, # 8 p. 2766 - 2779 |
|
~% 
Boc-His-OH CAS#:17791-52-5 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 37, # 11 p. 3112 - 3113 |
|
~% 
Boc-His-OH CAS#:17791-52-5 |
| Literature: Chemistry of Natural Compounds, , vol. 18, # 3 p. 322 - 327 Khimiya Prirodnykh Soedinenii, , # 3 p. 349 - 354 |
|
~% 
Boc-His-OH CAS#:17791-52-5 |
| Literature: Bulletin des Societes Chimiques Belges, , vol. 99, # 10 p. 779 - 782 |
| Precursor 8 | |
|---|---|
| DownStream 7 | |
| HS Code | 2933290090 |
|---|---|
| Summary | 2933290090. other compounds containing an unfused imidazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Transport and signaling via the amino acid binding site of the yeast Gap1 amino acid transceptor.
Nat. Chem. Biol. 5 , 45-52, (2009) Transporter-related nutrient sensors, called transceptors, mediate nutrient activation of signaling pathways through the plasma membrane. The mechanism of action of transporting and nontransporting tr... |
|
|
Exploring advantages/disadvantages and improvements in overcoming gene delivery barriers of amino acid modified trimethylated chitosan.
Pharm. Res. 32 , 2038-50, (2015) Present study aimed at exploring advantages/disadvantages of amino acid modified trimethylated chitosan in conquering multiple gene delivery obstacles and thus providing comprehensive understandings f... |
| tert-Butyloxycarbonyl-L-histidine |
| Nalpha-BOC-L-Histidine |
| n|A-boc-l-histidine |
| MFCD00065576 |
| Na-(tert-Butoxycarbonyl)-L-histidine |
| Boc-His-OH |
| N-{[(2-Methyl-2-propanyl)oxy]carbonyl}-L-histidine |
| N-Boc-histidine |
| Nα-(tert-Butoxycarbonyl)-L-histidine |
| L-Histidine, N-[(1,1-dimethylethoxy)carbonyl]- |
| Nalpha-(tert-Butoxycarbonyl)-L-histidine |
| Boc-L-Histidine |
| N-(tert-Butoxycarbonyl)-L-histidine |
| EINECS 241-768-9 |
| N-Boc-L-histidine |
| Nα-boc-L-histidine |



 CAS#:32926-43-5
CAS#:32926-43-5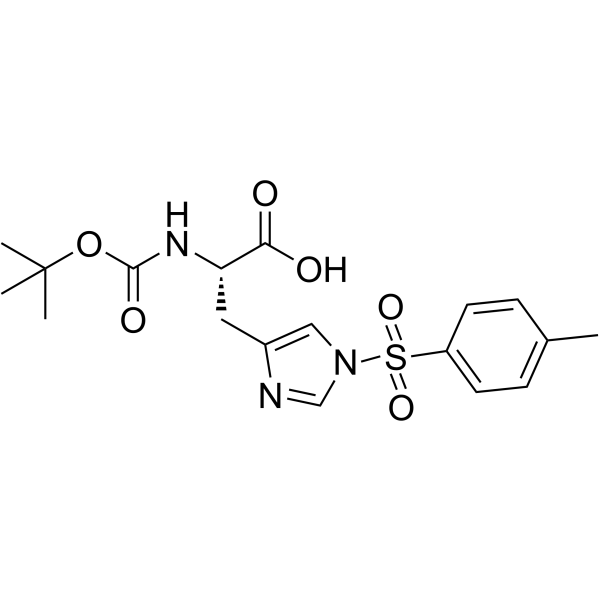 CAS#:35899-43-5
CAS#:35899-43-5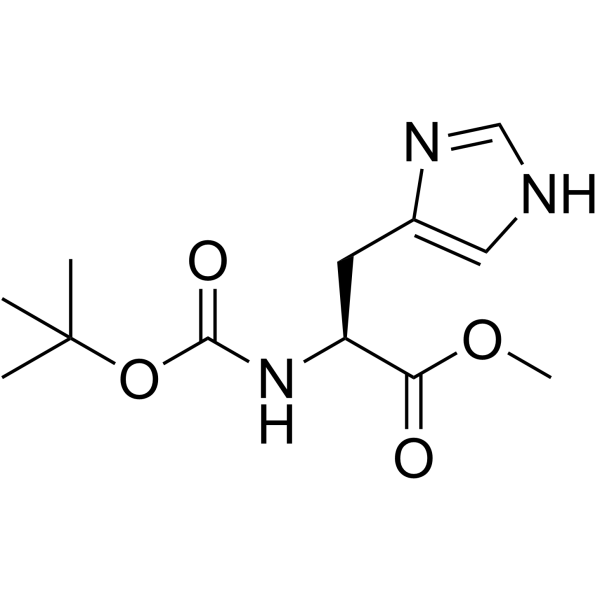 CAS#:2488-14-4
CAS#:2488-14-4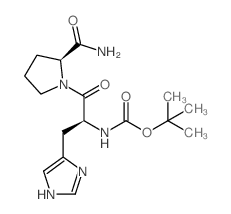 CAS#:29133-55-9
CAS#:29133-55-9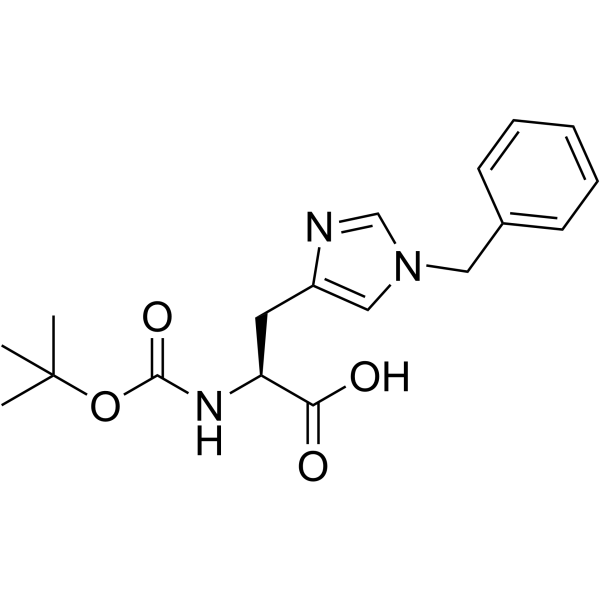 CAS#:20898-44-6
CAS#:20898-44-6![2-benzyl-N-[1-[[3-cyclohexyl-1-(3-ethyl-2-oxo-1,3-oxazolidin-5-yl)-1-hydroxypropan-2-yl]amino]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]-N'-[2-(2-methoxyethoxymethoxy)ethyl]-N'-methylbutanediamide structure](https://image.chemsrc.com/caspic/169/122224-84-4.png) CAS#:122224-84-4
CAS#:122224-84-4 CAS#:61070-20-0
CAS#:61070-20-0
