Tryptoline

Tryptoline structure
|
Common Name | Tryptoline | ||
|---|---|---|---|---|
| CAS Number | 16502-01-5 | Molecular Weight | 172.226 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 351.6±32.0 °C at 760 mmHg | |
| Molecular Formula | C11H12N2 | Melting Point | 206-208 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 166.5±25.1 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of TryptolineTetrahydro-β-carboline (Tryptoline) is a metabolite of tryptamine, also is a competitive serotonin reuptake inhibitor with an Ki value of 6.1 µM[1]. |
| Name | 1,2,3,4-tetrahydro-9h-pyrido[3,4-b]indole |
|---|---|
| Synonym | More Synonyms |
| Description | Tetrahydro-β-carboline (Tryptoline) is a metabolite of tryptamine, also is a competitive serotonin reuptake inhibitor with an Ki value of 6.1 µM[1]. |
|---|---|
| Related Catalog | |
| Target |
serotonin:6.1 μM (Ki) |
| In Vivo | Tetrahydro-β-carboline (20 µg; i.c.v.) increases the serotonin levels in the same part of the brain in rats[1]. Animal Model: 180-200g female Sprague-Dawley rats[1] Dosage: 20 µg Administration: Intraventricular injection Result: Increased of serotonin levels in the same part of the brain whereas the monoamine oxidase activity was not altered. |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 351.6±32.0 °C at 760 mmHg |
| Melting Point | 206-208 °C(lit.) |
| Molecular Formula | C11H12N2 |
| Molecular Weight | 172.226 |
| Flash Point | 166.5±25.1 °C |
| Exact Mass | 172.100052 |
| PSA | 27.82000 |
| LogP | 1.33 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.670 |
| Storage condition | Refrigerator |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Fingerprint analysis of thermolytic decarboxylation of tryptophan to tryptamine catalyzed by natural oils.
J. Chromatogr. A. 1210(1) , 115-20, (2008) A number of N,N-dialkylated tryptamines show psychoactive properties in man which resulted in a renewed interest in psychopharmacological research. Attempts to manufacture these derivatives are increa... |
|
|
Biosynthetic gene cluster for the cladoniamides, bis-indoles with a rearranged scaffold.
PLoS ONE 6(8) , e23694, (2011) The cladoniamides are bis-indole alkaloids isolated from Streptomyces uncialis, a lichen-associated actinomycete strain. The cladoniamides have an unusual, indenotryptoline structure rarely observed a... |
|
|
A class of oral N-[(1S,3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carbonyl]- N'-(amino-acid-acyl)hydrazine: discovery, synthesis, in vitro anti-platelet aggregation/in vivo anti-thrombotic evaluation and 3D QSAR analysis.
Eur. J. Med. Chem. 46 , 3237-49, (2011) The in vivo anti-thrombotic activities of amino acid modified tetrahydro-β-carbolines depended upon the proximity of the side chain of the amino acid residue to the carboline-cycle. Based on this prox... |
| 2,3,4,9-Tetrahydro-1H-β-carboline |
| Tetrahydro-b-carboline |
| Tetrahydro-β-carboline |
| 1,2,3,4-Tetrahydro-b-carboline |
| 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole |
| 1H-Pyrido[3,4-b]indole, 2,3,4,9-tetrahydro- |
| MFCD00004954 |
| 1,2,3,4-Tetrahydro-9H-Pyrido[3,4-B]Indole |
| Tetrahydro-beta-carboline |
| Triptoline |
| Tryptoline |
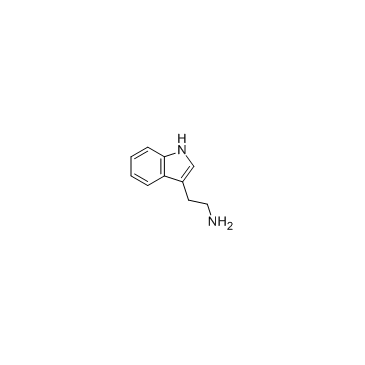 CAS#:61-54-1
CAS#:61-54-1 CAS#:298-12-4
CAS#:298-12-4 CAS#:50-00-0
CAS#:50-00-0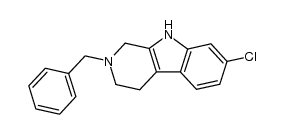 CAS#:606926-46-9
CAS#:606926-46-9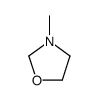 CAS#:27970-32-7
CAS#:27970-32-7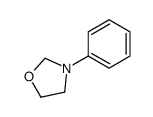 CAS#:20503-92-8
CAS#:20503-92-8 CAS#:14558-49-7
CAS#:14558-49-7 CAS#:3612-20-2
CAS#:3612-20-2 CAS#:126781-38-2
CAS#:126781-38-2![1-phenyl-2-(1,3,4,9-tetrahydropyrido[3,4-b]indol-2-yl)ethanone structure](https://image.chemsrc.com/caspic/288/111733-93-8.png) CAS#:111733-93-8
CAS#:111733-93-8 CAS#:84-26-4
CAS#:84-26-4 CAS#:244-63-3
CAS#:244-63-3 CAS#:4894-26-2
CAS#:4894-26-2![7-Nitro-1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole structure](https://image.chemsrc.com/caspic/196/642412-39-3.png) CAS#:642412-39-3
CAS#:642412-39-3![TERT-BUTYL 3,4-DIHYDRO-1H-PYRIDO[3,4-B]INDOLE-2(9H)-CARBOXYLATE structure](https://image.chemsrc.com/caspic/168/168824-94-0.png) CAS#:168824-94-0
CAS#:168824-94-0![1,2,3,4-TETRAHYDRO-9-(PHENYLMETHYL)-PYRIDO[3,4-B]INDOLE structure](https://image.chemsrc.com/caspic/131/134331-71-8.png) CAS#:134331-71-8
CAS#:134331-71-8 CAS#:121911-03-3
CAS#:121911-03-3 CAS#:13100-00-0
CAS#:13100-00-0![9-Methyl-9H-Pyrido[3,4-b]indole hydrochloride structure](https://image.chemsrc.com/caspic/196/752213-27-7.png) CAS#:752213-27-7
CAS#:752213-27-7
