D-tryptophan
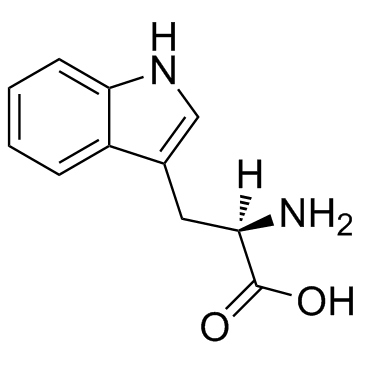
D-tryptophan structure
|
Common Name | D-tryptophan | ||
|---|---|---|---|---|
| CAS Number | 153-94-6 | Molecular Weight | 204.225 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 447.9±35.0 °C at 760 mmHg | |
| Molecular Formula | C11H12N2O2 | Melting Point | 282-285ºC | |
| MSDS | Chinese USA | Flash Point | 224.7±25.9 °C | |
Use of D-tryptophanH-D-Trp-OH is a D-stereoisomer of tryptophan and occasionally found in naturally produced peptides such as the marine venom peptide. |
| Name | D-tryptophan |
|---|---|
| Synonym | More Synonyms |
| Description | H-D-Trp-OH is a D-stereoisomer of tryptophan and occasionally found in naturally produced peptides such as the marine venom peptide. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 447.9±35.0 °C at 760 mmHg |
| Melting Point | 282-285ºC |
| Molecular Formula | C11H12N2O2 |
| Molecular Weight | 204.225 |
| Flash Point | 224.7±25.9 °C |
| Exact Mass | 204.089874 |
| PSA | 79.11000 |
| LogP | 1.04 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.698 |
| InChIKey | QIVBCDIJIAJPQS-UHFFFAOYSA-N |
| SMILES | NC(Cc1c[nH]c2ccccc12)C(=O)O |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Enantioseparation of (DL)-tryptophan by spiral tube assembly counter-current chromatography and evaluation of mass transfer rate for enantiomers.
J. Chromatogr. A. 1374 , 77-84, (2014) Spiral tube assembly counter-current chromatography was successfully applied in enantioseparation of dl-tryptophan using bovine serum albumin as chiral selector. An improved biphasic aqueous-aqueous s... |
|
|
Inhibition of Pseudomonas aeruginosa biofilm formation on wound dressings.
Wound Repair Regen. 23 , 842-54, (2015) Chronic nonhealing skin wounds often contain bacterial biofilms that prevent normal wound healing and closure and present challenges to the use of conventional wound dressings. We investigated inhibit... |
|
|
Carbonic anhydrase activators: activation of the human tumor-associated isozymes IX and XII with amino acids and amines.
Bioorg. Med. Chem. 16 , 3530-6, (2008) The first activation study of the human carbonic anhydrase (hCA, EC 4.2.1.1) isoforms associated to tumors, hCA IX and XII, with a small library of natural and non-natural amino acids as well as aroma... |
| (2R)-2-amino-3-(1H-indol-3-yl)propanoic acid |
| (R)-a-Amino-3-indolepropionic Acid |
| Tryptophan |
| H-D-Trp-OH |
| (R)-a-Aminoindole-3-propanoic Acid |
| EINECS 205-819-9 |
| D-α-Amino-3-indolepropionic acid |
| (R)-tryptophan |
| D-tryptophan |
| D-tryptophan zwitterion |
| (+)-Tryptophan |
| D(+)-Tryptophan |
| tryptophane |
| MFCD00005647 |
| (R)-2-Amino-3-(3-indolyl)propionic acid |
| (R)-(+)-2-Amino-3-(3-indolyl)propionic Acid |
| Tryptophan, D- |
| Tadalafil Impurity 1 |
 CAS#:54-12-6
CAS#:54-12-6 CAS#:338-69-2
CAS#:338-69-2 CAS#:392-12-1
CAS#:392-12-1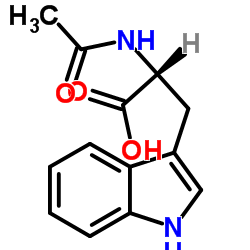 CAS#:2280-01-5
CAS#:2280-01-5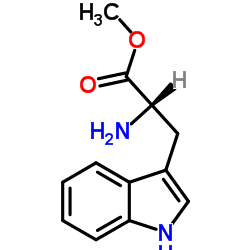 CAS#:4299-70-1
CAS#:4299-70-1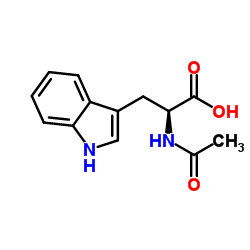 CAS#:1218-34-4
CAS#:1218-34-4 CAS#:66872-76-2
CAS#:66872-76-2 CAS#:742100-62-5
CAS#:742100-62-5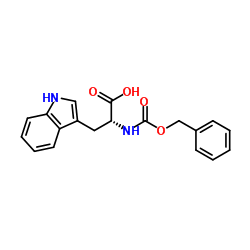 CAS#:2279-15-4
CAS#:2279-15-4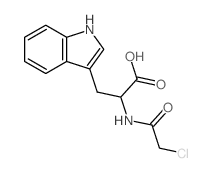 CAS#:79189-76-7
CAS#:79189-76-7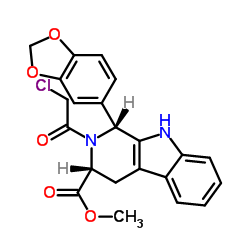 CAS#:171489-59-1
CAS#:171489-59-1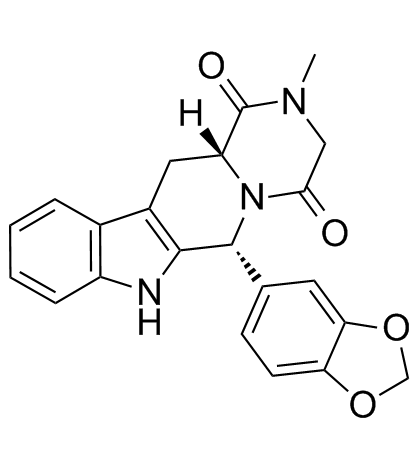 CAS#:171596-29-5
CAS#:171596-29-5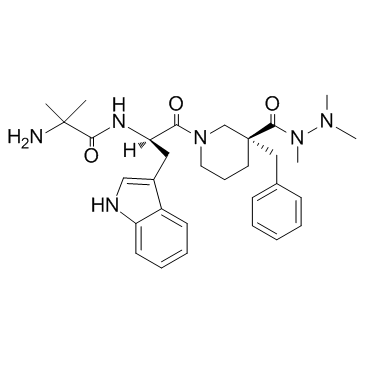 CAS#:249921-19-5
CAS#:249921-19-5 CAS#:72002-54-1
CAS#:72002-54-1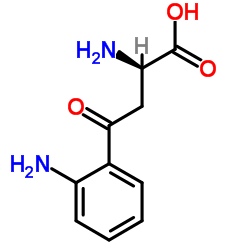 CAS#:13441-51-5
CAS#:13441-51-5 CAS#:100-02-7
CAS#:100-02-7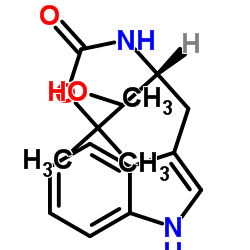 CAS#:158932-00-4
CAS#:158932-00-4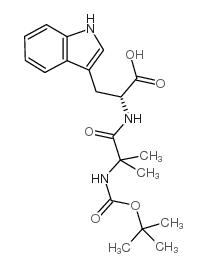 CAS#:159634-94-3
CAS#:159634-94-3
