Ro 46-2005
Modify Date: 2024-01-16 11:47:48
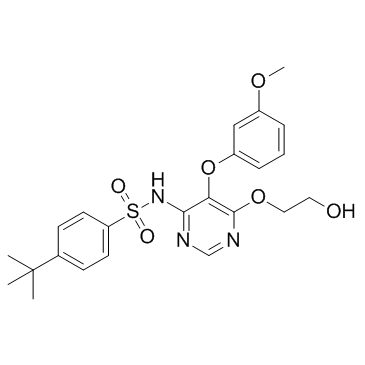
Ro 46-2005 structure
|
Common Name | Ro 46-2005 | ||
|---|---|---|---|---|
| CAS Number | 150725-87-4 | Molecular Weight | 473.542 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 617.5±65.0 °C at 760 mmHg | |
| Molecular Formula | C23H27N3O6S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 327.3±34.3 °C | |
Use of Ro 46-2005Ro 46-2005 is a novel synthetic non-peptide endothelin receptor antagonist, inhibits the specific binding of 125I-ET-1 to human vascular smooth muscle cells (ETA receptor) with IC50 of 220 nM.IC50 value: 220 nM (ETA) [2]Target: Endothelinin vitro: Ro 46-2005 proves to be equipotent (IC50 200-500 nM) for inhibition of [125I]ET-1 binding on the two known ET receptor subtypes (ETA and ETB). Ro 46-2005 also inhibits the functional consequences of ET-1 stimulation: the ET-l-induced release of arachidonic acid from rat mesangial cells was inhibited with an IC50 of 1.8 μM.[1] |
| Name | 4-tert-butyl-N-[6-(2-hydroxyethoxy)-5-(3-methoxyphenoxy)pyrimidin-4-yl]benzenesulfonamide |
|---|---|
| Synonym | More Synonyms |
| Description | Ro 46-2005 is a novel synthetic non-peptide endothelin receptor antagonist, inhibits the specific binding of 125I-ET-1 to human vascular smooth muscle cells (ETA receptor) with IC50 of 220 nM.IC50 value: 220 nM (ETA) [2]Target: Endothelinin vitro: Ro 46-2005 proves to be equipotent (IC50 200-500 nM) for inhibition of [125I]ET-1 binding on the two known ET receptor subtypes (ETA and ETB). Ro 46-2005 also inhibits the functional consequences of ET-1 stimulation: the ET-l-induced release of arachidonic acid from rat mesangial cells was inhibited with an IC50 of 1.8 μM.[1] |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 617.5±65.0 °C at 760 mmHg |
| Molecular Formula | C23H27N3O6S |
| Molecular Weight | 473.542 |
| Flash Point | 327.3±34.3 °C |
| Exact Mass | 473.162048 |
| PSA | 128.25000 |
| LogP | 2.22 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.593 |
| Storage condition | 2-8℃ |
|
~% 
Ro 46-2005 CAS#:150725-87-4 |
| Literature: Hoffmann-La Roche Inc. Patent: US5292740 A1, 1994 ; US 5292740 A |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| Benzenesulfonamide, 4-(1,1-dimethylethyl)-N-[6-(2-hydroxyethoxy)-5-(3-methoxyphenoxy)-4-pyrimidinyl]- |
| N-[6-(2-Hydroxyethoxy)-5-(3-methoxyphenoxy)-4-pyrimidinyl]-4-(2-methyl-2-propanyl)benzenesulfonamide |
| Ro 46-2005 |
| 4-tert-butyl-N-[6-(2-hydroxyethoxy)-5-(3-methoxyphenoxy)pyrimidin-4-yl]benzenesulfonamide |