CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
UY0704600
-
CAS REGISTRY NUMBER :
-
14976-57-9
-
LAST UPDATED :
-
199203
-
DATA ITEMS CITED :
-
8
-
MOLECULAR FORMULA :
-
C21-H26-Cl-N-O.C4-H4-O4
-
MOLECULAR WEIGHT :
-
460.01
-
WISWESSER LINE NOTATION :
-
T5NTJ A1 B2OX1&R&R DG &QV1U1VQ -T
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
3550 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - excitement Behavioral - changes in motor activity (specific assay)
-
REFERENCE :
-
BCFAAI Bollettino Chimico Farmaceutico. (Societa Editoriale Farmaceutica, Via Ausonio 12, 20123 Milan, Italy) V.33- 1894- Volume(issue)/page/year: 106,467,1967
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
82 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - excitement Behavioral - changes in motor activity (specific assay)
-
REFERENCE :
-
BCFAAI Bollettino Chimico Farmaceutico. (Societa Editoriale Farmaceutica, Via Ausonio 12, 20123 Milan, Italy) V.33- 1894- Volume(issue)/page/year: 106,467,1967
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
730 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - excitement Behavioral - changes in motor activity (specific assay)
-
REFERENCE :
-
BCFAAI Bollettino Chimico Farmaceutico. (Societa Editoriale Farmaceutica, Via Ausonio 12, 20123 Milan, Italy) V.33- 1894- Volume(issue)/page/year: 106,467,1967
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
43 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - excitement Behavioral - changes in motor activity (specific assay)
-
REFERENCE :
-
BCFAAI Bollettino Chimico Farmaceutico. (Societa Editoriale Farmaceutica, Via Ausonio 12, 20123 Milan, Italy) V.33- 1894- Volume(issue)/page/year: 106,467,1967
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
175 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - hallucinations, distorted perceptions Behavioral - muscle contraction or spasticity Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
BCFAAI Bollettino Chimico Farmaceutico. (Societa Editoriale Farmaceutica, Via Ausonio 12, 20123 Milan, Italy) V.33- 1894- Volume(issue)/page/year: 106,467,1967
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
>50 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - hallucinations, distorted perceptions Behavioral - muscle contraction or spasticity Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
BCFAAI Bollettino Chimico Farmaceutico. (Societa Editoriale Farmaceutica, Via Ausonio 12, 20123 Milan, Italy) V.33- 1894- Volume(issue)/page/year: 106,467,1967
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
1 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: 6,230,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
19 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - excitement Behavioral - changes in motor activity (specific assay)
-
REFERENCE :
-
BCFAAI Bollettino Chimico Farmaceutico. (Societa Editoriale Farmaceutica, Via Ausonio 12, 20123 Milan, Italy) V.33- 1894- Volume(issue)/page/year: 106,467,1967
|

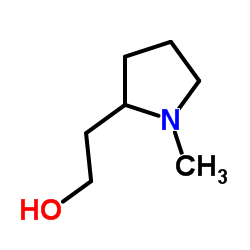 CAS#:67004-64-2
CAS#:67004-64-2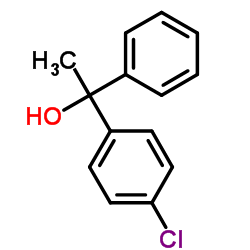 CAS#:59767-24-7
CAS#:59767-24-7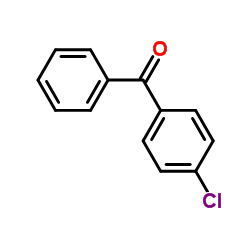 CAS#:134-85-0
CAS#:134-85-0