thiethylperazine
Modify Date: 2025-08-25 06:32:25
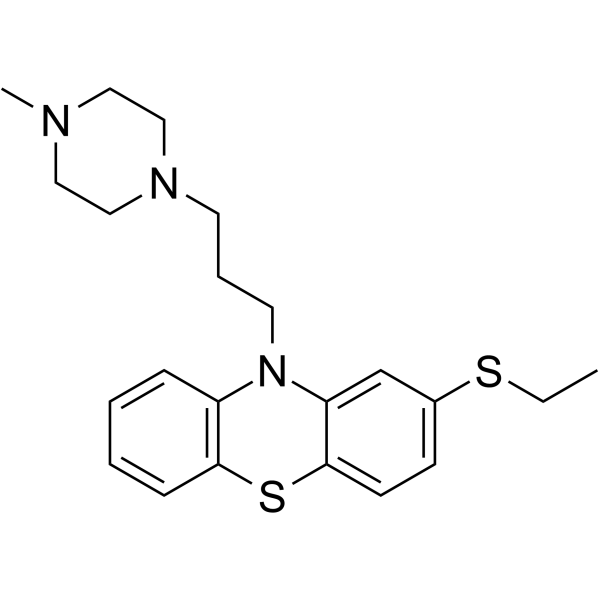
thiethylperazine structure
|
Common Name | thiethylperazine | ||
|---|---|---|---|---|
| CAS Number | 1420-55-9 | Molecular Weight | 399.61600 | |
| Density | 1.24g/cm3 | Boiling Point | 559.8ºC at 760mmHg | |
| Molecular Formula | C22H29N3S2 | Melting Point | 62-64° | |
| MSDS | N/A | Flash Point | 292.4ºC | |
Use of thiethylperazineThiethylperazine, a phenothiazine derivate, is an orally active and potent dopamine D2-receptor and histamine H1-receptor antagonist. Thiethylperazine is also a selective ABCC1activator that reduces amyloid-β (Aβ) load in mice. Thiethylperazine has anti-emetic, antipsychotic and antimicrobial effects[1][2][3]. |
| Name | thiethylperazine |
|---|---|
| Synonym | More Synonyms |
| Description | Thiethylperazine, a phenothiazine derivate, is an orally active and potent dopamine D2-receptor and histamine H1-receptor antagonist. Thiethylperazine is also a selective ABCC1activator that reduces amyloid-β (Aβ) load in mice. Thiethylperazine has anti-emetic, antipsychotic and antimicrobial effects[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
D2 Receptor H1 Receptor |
| In Vitro | Thiethylperazine can enhance the antibiotic (Vancomycin) activity at a concentration as low as 2 μg/mL. Thiethylperazine inhibits Vancomycin-sensitive E. faecalis ATCC 29212, Vancomycin-resistant E. faecalis ATCC 51299 and vancomycin-resistant E. faecalis (VREF) isolates with MIC values of 8 μg/mL, 16 μg/mL and 8 μg/mL, respectively[3]. |
| In Vivo | Thiethylperazine (3 mg/kg; intramuscular injection; twice daily; for 30 days) significantly reduces Aβ42 levels in young APP/PS1 mice[2]. Animal Model: Young Aβ precursor protein (APPswe) and mutant presenilin-1 (PS1) (APP/PS1) mice[2] Dosage: 3 mg/kg Administration: Intramuscular injection; twice daily; for 30 days Result: Significantly reduced Aβ42 levels in APP/PS1 mice. |
| References |
| Density | 1.24g/cm3 |
|---|---|
| Boiling Point | 559.8ºC at 760mmHg |
| Melting Point | 62-64° |
| Molecular Formula | C22H29N3S2 |
| Molecular Weight | 399.61600 |
| Flash Point | 292.4ºC |
| Exact Mass | 399.18000 |
| PSA | 60.32000 |
| LogP | 4.97960 |
| Index of Refraction | 1.5605 (estimate) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Ethylthioperazine. |
| Torecane |
| Tietylperazine |
| thietylperazine |