N-Boc-L-lysine
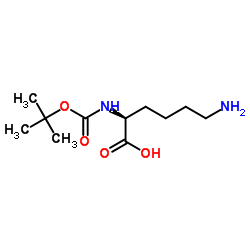
N-Boc-L-lysine structure
|
Common Name | N-Boc-L-lysine | ||
|---|---|---|---|---|
| CAS Number | 13734-28-6 | Molecular Weight | 246.30 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 410.5±40.0 °C at 760 mmHg | |
| Molecular Formula | C11H22N2O4 | Melting Point | ~205 °C (dec.)(lit.) | |
| MSDS | USA | Flash Point | 202.0±27.3 °C | |
Use of N-Boc-L-lysineBoc-Lys-OH is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | N-alpha-(tert-Butoxycarbonyl)-L-lysine |
|---|---|
| Synonym | More Synonyms |
| Description | Boc-Lys-OH is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 410.5±40.0 °C at 760 mmHg |
| Melting Point | ~205 °C (dec.)(lit.) |
| Molecular Formula | C11H22N2O4 |
| Molecular Weight | 246.30 |
| Flash Point | 202.0±27.3 °C |
| Exact Mass | 246.157959 |
| PSA | 101.65000 |
| LogP | 0.74 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.485 |
| Storage condition | 2~8°C |
| Precursor 9 | |
|---|---|
| DownStream 8 | |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Transport and signaling via the amino acid binding site of the yeast Gap1 amino acid transceptor.
Nat. Chem. Biol. 5 , 45-52, (2009) Transporter-related nutrient sensors, called transceptors, mediate nutrient activation of signaling pathways through the plasma membrane. The mechanism of action of transporting and nontransporting tr... |
|
|
A Mannose Family Phosphotransferase System Permease and Associated Enzymes Are Required for Utilization of Fructoselysine and Glucoselysine in Salmonella enterica Serovar Typhimurium.
J. Bacteriol. 197 , 2831-9, (2015) Salmonella enteric serovar Typhimurium, a major cause of food-borne illness, is capable of using a variety of carbon and nitrogen sources. Fructoselysine and glucoselysine are Maillard reaction produc... |
|
|
Synthesis and evaluation of a targeted nanoglobular dual-modal imaging agent for MR imaging and image-guided surgery of prostate cancer.
Pharm. Res. 31(6) , 1469-76, (2014) To synthesize and evaluate a peptide targeted nanoglobular dual modal imaging agent specific to a cancer biomarker in tumor stroma for MRI and fluorescence visualization of prostate tumor in image-gui... |
| Nα-Boc-L-Lysine |
| N(Alpha)-Boc-L-Lysine |
| Nα-(Tert-Butoxycarbonyl)-L-Lysine |
| N-{[(2-Methyl-2-propanyl)oxy]carbonyl}-L-lysine |
| (S)-2-Amino-6-((tert-butoxycarbonyl)amino)hexanoic acid |
| L-Lysine, N-[(1,1-dimethylethoxy)carbonyl]- |
| N-a-(tert.-butoxycarbonyl)-L-lysine |
| MFCD00038203 |
| EINECS 237-303-4 |
| N-α-Boc-L-lysine |
| L-Lysine, N6-((1,1-dimethylethoxy)carbonyl)- |
| N2-((1,1-Dimethylethoxy)carbonyl)-L-lysine |
| N-Boc lysine |
| N-α-t-Boc-L-lysine |
| Boc-D-Lys-OH |
| N-(tert-Butoxycarbonyl)-L-lysine |
| N-α-(tert-Butoxycarbonyl)-L-lysine |
| Boc-Lys-OH |
| Boc-L-lysine |
| N-Boc-L-lysine |
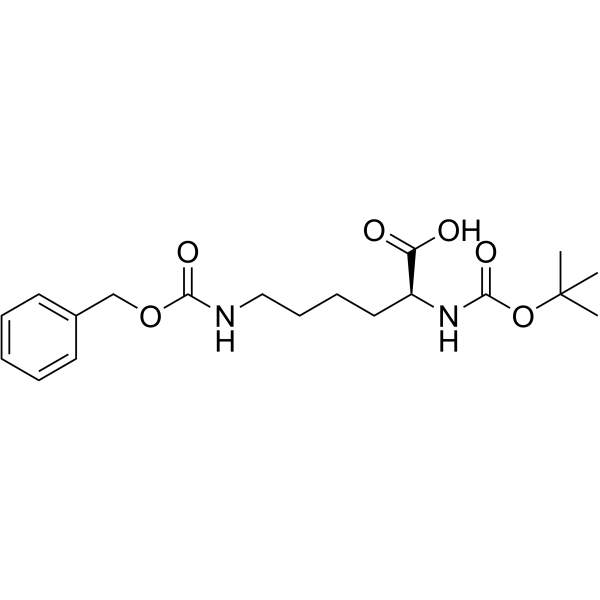 CAS#:2389-45-9
CAS#:2389-45-9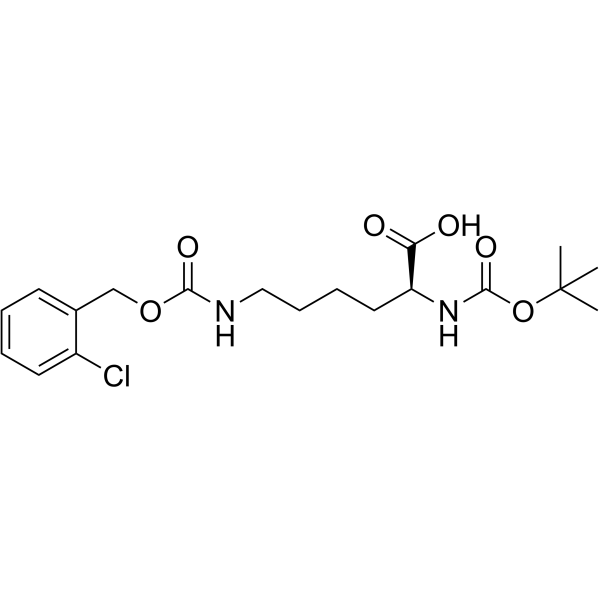 CAS#:54613-99-9
CAS#:54613-99-9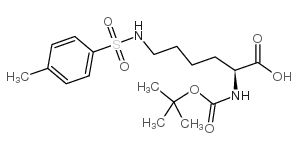 CAS#:13734-29-7
CAS#:13734-29-7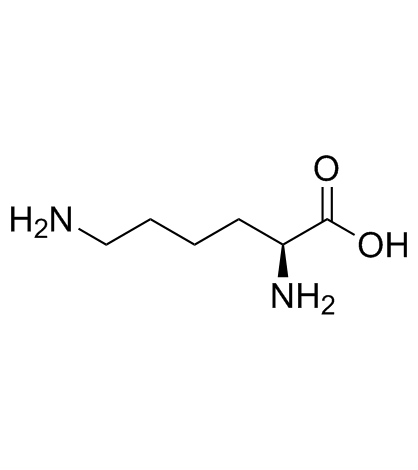 CAS#:56-87-1
CAS#:56-87-1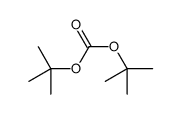 CAS#:34619-03-9
CAS#:34619-03-9 CAS#:77236-13-6
CAS#:77236-13-6 CAS#:24424-99-5
CAS#:24424-99-5![L-Lysine,N6-[(4-methylphenyl)sulfonyl]- Structure](https://image.chemsrc.com/caspic/080/2130-76-9.png) CAS#:2130-76-9
CAS#:2130-76-9 CAS#:1155-64-2
CAS#:1155-64-2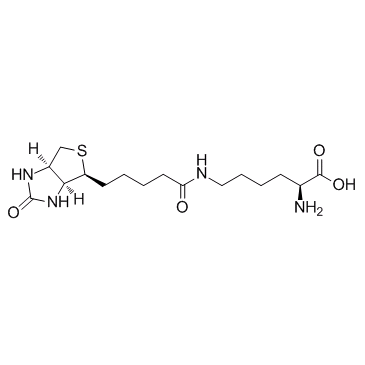 CAS#:576-19-2
CAS#:576-19-2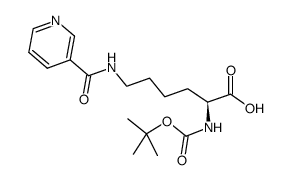 CAS#:14609-04-2
CAS#:14609-04-2 CAS#:50-70-4
CAS#:50-70-4 CAS#:2483-47-8
CAS#:2483-47-8 CAS#:79839-29-5
CAS#:79839-29-5 CAS#:77611-37-1
CAS#:77611-37-1 CAS#:69-65-8
CAS#:69-65-8 CAS#:6404-26-8
CAS#:6404-26-8
