Xenon difluoride
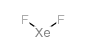
Xenon difluoride structure
|
Common Name | Xenon difluoride | ||
|---|---|---|---|---|
| CAS Number | 13709-36-9 | Molecular Weight | 169.29000 | |
| Density | 4.32 g/mL at 25 °C(lit.) | Boiling Point | N/A | |
| Molecular Formula | F2Xe | Melting Point | 129 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | None | |
| Symbol |



GHS03, GHS05, GHS06 |
Signal Word | Danger | |
| Name | xenon difluoride |
|---|---|
| Synonym | More Synonyms |
| Density | 4.32 g/mL at 25 °C(lit.) |
|---|---|
| Melting Point | 129 °C(lit.) |
| Molecular Formula | F2Xe |
| Molecular Weight | 169.29000 |
| Flash Point | None |
| Exact Mass | 169.90100 |
| LogP | 0.45960 |
| Vapour Pressure | 3.8 mm Hg ( 25 °C) |
Synonym: Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
Risk Phrases: 36/37/38 Section 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.Hygroscopic. Potential Health Effects Eye: Dust may cause mechanical irritation. Skin: Dust may cause mechanical irritation. Effects of contact may be delayed. Ingestion: May cause digestive tract disturbances. Inhalation: May cause respiratory tract irritation. Effects of inhalation may be delayed. Chronic: No information found. Section 4 - FIRST AID MEASURES Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately. Skin: Get medical aid. Flush skin with plenty of soap and water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Ingestion: If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid immediately. Inhalation: Remove from exposure to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Notes to Physician: Treat symptomatically and supportively. Section 5 - FIRE FIGHTING MEASURES General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Substance is noncombustible. Containers may explode when heated. Extinguishing Media: Do NOT use straight streams of water. For small fires, use dry chemical, carbon dioxide, or water spray. For large fires, use water spray, fog or regular foam. Cool containers with flooding quantities of water until well after fire is out. Section 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8. Spills/Leaks: Avoid runoff into storm sewers and ditches which lead to waterways. Wash area with soap and water. Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Avoid generating dusty conditions. Provide ventilation. Do not get water inside containers. Section 7 - HANDLING and STORAGE Handling: Wash thoroughly after handling. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Storage: Store in a cool, dry, well-ventilated area away from incompatible substances. Keep containers tightly closed. Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls: Use adequate ventilation to keep airborne concentrations low. Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166. Skin: Wear appropriate protective gloves to prevent skin exposure. Clothing: Wear appropriate protective clothing to prevent skin exposure. Respirators: Follow the OSHA respirator regulations found in 29CFR 1910.134 or European Standard EN 149. Always use a NIOSH or European Standard EN 149 approved respirator when necessary. Section 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Powder Appearance: off-white Odor: odorless pH: Not available. Vapor Pressure: Not available. Viscosity: Not available. Boiling Point: Not available. Freezing/Melting Point: Not available. Autoignition Temperature: Not available. Flash Point: Not available. Explosion Limits, lower: Not available. Explosion Limits, upper: Not available. Decomposition Temperature: Solubility in water: insoluble Specific Gravity/Density: 5.9360g/cm3 Molecular Formula: F3La Molecular Weight: 195.91 Section 10 - STABILITY AND REACTIVITY Chemical Stability: Stable under normal temperatures and pressures. Conditions to Avoid: Incompatible materials, dust generation, moisture. Incompatibilities with Other Materials: Strong oxidizing agents, strong acids. Hazardous Decomposition Products: Irritating and toxic fumes and gases, hydrogen fluoride, fluorine. Hazardous Polymerization: Will not occur. Section 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 13709-36-9: ZE1294166 LD50/LC50: CAS# 13709-36-9: Inhalation, mouse: LC50 = 445 mg/m3/2H; Oral, mouse: LD50 = 90 mg/kg. Carcinogenicity: Xenon difluoride - ACGIH: A4 - Not Classifiable as a Human Carcinogen (as F) (listed See actual entry in RTECS for complete information. Section 12 - ECOLOGICAL INFORMATION Section 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations. Section 14 - TRANSPORT INFORMATION IATA No information available. IMO No information available. RID/ADR No information available. Section 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives Hazard Symbols: XI Risk Phrases: R 36/37/38 Irritating to eyes, respiratory system and skin. Safety Phrases: S 26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 37/39 Wear suitable gloves and eye/face protection. WGK (Water Danger/Protection) CAS# 13709-36-9: No information available. United Kingdom Occupational Exposure Limits CAS# 13709-36-9: OES-United Kingdom, TWA (listed as ** undefined **): as F: 2.5 mg/m3 TWA Canada CAS# 13709-36-9 is listed on Canada's NDSL List. CAS# 13709-36-9 is not listed on Canada's Ingredient Disclosure List. Exposure Limits CAS# 13709-36-9 (listed as ** undefined **): OEL-AUSTRALIA:TWA 2.5 mg( F)/m3 OEL-BELGIUM:TWA 2.5 mg(F)/m3 OEL-CZECHOSLOVAKIA:TWA 1 mg(F)/m3;STEL 5 mg(F)/m3 OEL-DENMARK:TWA 2.5 mg(F)/m3 OEL-FINLAND:TWA 2.5 mg(F)/m3 OEL-FRANCE:TWA 2.5 mg(F)/m3 OEL-GERMANY:TWA 2.5 mg(F)/m3 OEL-HUNGARY:TWA 1 mg(F)/m3;STEL 2 mg(F)/m3 OEL-THE NETHERLANDS:TWA 2.5 mg(F)/m3 OEL-THE PHILIPPINES:TWA 2.5 mg(F)/m3 OEL-POLAND:TWA 1 mg(F)/m3 OEL-SWEDEN:TWA 2 mg(F)/m3 OEL-SWITZERLAND:TWA 1.8 ppm (1.5 mg(F)/m3);STEL 9.0 ppm OEL-THAILAND:TWA 2.5 mg(F)/m3 OEL-TURKEY:TWA 2.5 mg(F)/m3 OEL-UNITED KINGDOM:TWA 2.5 mg(F)/m3 US FEDERAL TSCA CAS# 13709-36-9 is listed on the TSCA inventory. SECTION 16 - ADDITIONAL INFORMATION N/A |
| Symbol |



GHS03, GHS05, GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H272-H301-H314-H330 |
| Precautionary Statements | Missing Phrase - N15.00950417-P210-P220-P280-P304 + P340 + P310-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | O,T+,T |
| Risk Phrases | R8:Contact with combustible material may cause fire. R25:Toxic if swallowed. R26:Very Toxic by inhalation. R34:Causes burns. |
| Safety Phrases | S17-S26-S28-S36/37/39-S45 |
| RIDADR | UN 3087 5.1/PG 2 |
| WGK Germany | 3 |
| RTECS | ZE1294166 |
| Packaging Group | II |
| Hazard Class | 5.1 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
|
Synthesis of α,α-difluoroethyl aryl and heteroaryl ethers.
Org. Lett. 14(15) , 3944-7, (2012) Fluorine plays a critical role in modern medicinal chemistry due to its unique properties, and new methods for its incorporation into target molecules are of high interest. An efficient new method for... |
|
|
Fluorination with XeF(2).(1) 44. Effect of Geometry and Heteroatom on the Regioselectivity of Fluorine Introduction into an Aromatic Ring.
J. Org. Chem. 63 , 878, (1998)
|
|
|
Xenon difluoride induced aryl iodide reductive elimination: a simple access to difluoropalladium(II) complexes.
Inorg. Chem. 47(1) , 5-7, (2008) Palladium(II) aryliodo complexes bearing chelating diphosphine ligands react with XeF2, giving iodoarene and rare palladium(II) difluoro complexes. The reaction is general with regard to the aryl grou... |
| difluoroxenon |
| MFCD00040538 |
| EINECS 237-251-2 |
| Xenon difluoride |
 CAS#:1160219-14-6
CAS#:1160219-14-6 CAS#:7664-39-3
CAS#:7664-39-3 CAS#:373-91-1
CAS#:373-91-1 CAS#:13774-85-1
CAS#:13774-85-1 CAS#:13776-58-4
CAS#:13776-58-4 CAS#:375-00-8
CAS#:375-00-8 CAS#:21308-45-2
CAS#:21308-45-2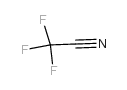 CAS#:353-85-5
CAS#:353-85-5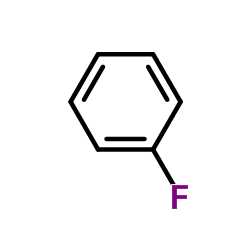 CAS#:462-06-6
CAS#:462-06-6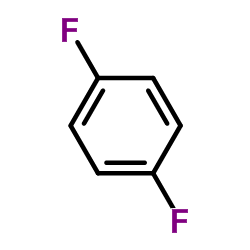 CAS#:540-36-3
CAS#:540-36-3 CAS#:367-11-3
CAS#:367-11-3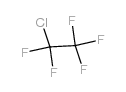 CAS#:76-15-3
CAS#:76-15-3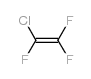 CAS#:79-38-9
CAS#:79-38-9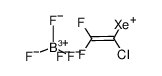 CAS#:726203-15-2
CAS#:726203-15-2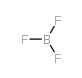 CAS#:7637-07-2
CAS#:7637-07-2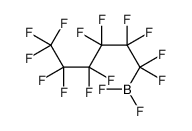 CAS#:329009-01-0
CAS#:329009-01-0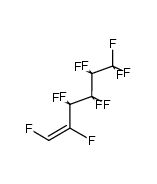 CAS#:391199-64-7
CAS#:391199-64-7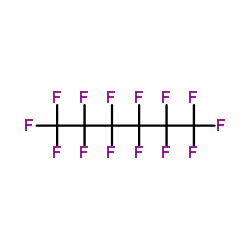 CAS#:355-42-0
CAS#:355-42-0