Cupferron
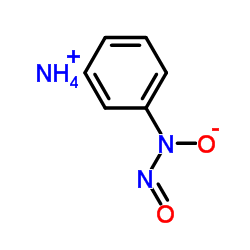
Cupferron structure
|
Common Name | Cupferron | ||
|---|---|---|---|---|
| CAS Number | 135-20-6 | Molecular Weight | 155.155 | |
| Density | N/A | Boiling Point | 243.8ºC at 760 mmHg | |
| Molecular Formula | C6H9N3O2 | Melting Point | 150-155 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 101.3ºC | |
| Symbol |


GHS06, GHS08 |
Signal Word | Danger | |
| Name | Cupferron |
|---|---|
| Synonym | More Synonyms |
| Boiling Point | 243.8ºC at 760 mmHg |
|---|---|
| Melting Point | 150-155 °C (dec.)(lit.) |
| Molecular Formula | C6H9N3O2 |
| Molecular Weight | 155.155 |
| Flash Point | 101.3ºC |
| Exact Mass | 155.069473 |
| PSA | 55.73000 |
| LogP | 2.04840 |
| Vapour Pressure | 0.0169mmHg at 25°C |
| InChIKey | GDEBSAWXIHEMNF-UHFFFAOYSA-O |
| SMILES | O=NN([O-])c1ccccc1.[NH4+] |
| Stability | Stable, but may be moisture sensitive. Incompatible with strong oxidizing agents, strong bases, strong acids. |
| Water Solubility | <0.1 g/100 mL at 18.5 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H315-H319-H335-H351 |
| Precautionary Statements | P261-P281-P301 + P310-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic; |
| Risk Phrases | R25;R36/37/38;R40 |
| Safety Phrases | S26-S36/37-S45 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | NC4725000 |
| Packaging Group | III |
| Hazard Class | 6.1 |
| HS Code | 3822009000 |
|
~% 
Cupferron CAS#:135-20-6 |
| Literature: Organic Preparations and Procedures International, , vol. 30, # 4 p. 439 - 446 |
|
~% 
Cupferron CAS#:135-20-6 |
| Literature: Organic Preparations and Procedures International, , vol. 30, # 4 p. 439 - 446 |
|
~% 
Cupferron CAS#:135-20-6 |
| Literature: Journal of the Chemical Society, , vol. 117, p. 589 |
|
~% 
Cupferron CAS#:135-20-6 |
| Literature: J.ind.eng.Chem., , vol. 12, p. 799 Chem. Zentralbl., , vol. 91, # IV p. 662 |
| HS Code | 2928000090 |
|---|---|
| Summary | 2928000090 other organic derivatives of hydrazine or of hydroxylamine VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:20.0% |
|
Intermediate analogue inhibitors of mandelate racemase: N-Hydroxyformanilide and cupferron.
Bioorg. Med. Chem. Lett. 17 , 105-8, (2007) Mandelate racemase (MR) catalyzes the 1,1-proton transfer that interconverts the enantiomers of mandelate. The transition state/intermediate analogues N-hydroxyformanilide (K(i)=2.79+/-0.19 microM) an... |
|
|
Celecoxib prodrugs possessing a diazen-1-ium-1,2-diolate nitric oxide donor moiety: synthesis, biological evaluation and nitric oxide release studies.
Bioorg. Med. Chem. Lett. 20 , 4544-9, (2010) A new class of anti-inflammatory (AI) cupferron prodrugs was synthesized wherein a diazen-1-ium-1,2-diolato ammonium salt, and its O(2)-methyl and O(2)-acetoxyethyl derivatives, nitric oxide (NO) dono... |
|
|
Synthesis and characterization of lithium oxonitrate (LiNO).
J. Inorg. Biochem. 118 , 128-33, (2013) The oxonitrate(1-) anion (NO(-)), the one-electron reduction product of nitric oxide and conjugate base of HNO, has not been synthesized and isolated due to the inherent reactivity of this anion. The ... |
| COPPERONE |
| CUPEFERRON |
| Kupferron |
| Cupferon |
| N-nitroso-N-phenyl-hydroxylamin,ammonium salt |
| EINECS 205-183-2 |
| Benzenamine, N-hydroxy-N-nitroso-, ammonium salt |
| Kupferon |
| CUPFERRON,ACS |
| N-hydroxy-N-nitroso-benzenamine,ammonium salt |
| Ammonium Nitrosophenylhydroxylamine |
| N-nitrosophenyl hydroxylamine ammonium salt |
| Ammonium 2-oxo-1-phenylhydrazinolate |
| MFCD00078422 |
| N-Nitroso-N-phenylhydroxylamine Ammonium Salt |
| N-Nitroso-N-phenyl-hydroxylamin,Ammonium-Salz |
 CAS#:495-48-7
CAS#:495-48-7
