N-Phenylhydroxylamine
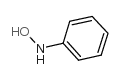
N-Phenylhydroxylamine structure
|
Common Name | N-Phenylhydroxylamine | ||
|---|---|---|---|---|
| CAS Number | 100-65-2 | Molecular Weight | 109.12600 | |
| Density | 1.214g/cm3 | Boiling Point | 215.8ºC at 760mmHg | |
| Molecular Formula | C6H7NO | Melting Point | 83 - 84ºC | |
| MSDS | Chinese USA | Flash Point | 120.2ºC | |
| Symbol |

GHS06 |
Signal Word | Danger | |
| Name | N-phenylhydroxylamine |
|---|---|
| Synonym | More Synonyms |
| Density | 1.214g/cm3 |
|---|---|
| Boiling Point | 215.8ºC at 760mmHg |
| Melting Point | 83 - 84ºC |
| Molecular Formula | C6H7NO |
| Molecular Weight | 109.12600 |
| Flash Point | 120.2ºC |
| Exact Mass | 109.05300 |
| PSA | 32.26000 |
| LogP | 1.56070 |
| Vapour Pressure | 0.085mmHg at 25°C |
| Index of Refraction | 1.649 |
| Storage condition | ?20°C |
| Stability | Unstable - deteriorates with storage. Incompatible with strong oxidizing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2928000090 |
|---|---|
| Summary | 2928000090 other organic derivatives of hydrazine or of hydroxylamine VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:20.0% |
|
In situ infrared monitoring of the solid/liquid catalyst interface during the three-phase hydrogenation of nitrobenzene over nanosized Au on TiO2.
Phys. Chem. Chem. Phys. 13(27) , 12463-71, (2011) The three-phase hydrogenation of nitrobenzene catalysed by nanosized gold over titania was investigated in a slurry. Simultaneous in situ ATR-FTIR monitoring of the liquid phase and at the solid/liqui... |
|
|
Genotoxic activities of aniline and its metabolites and their relationship to the carcinogenicity of aniline in the spleen of rats.
Crit. Rev. Toxicol. 35(10) , 783-835, (2005) Aniline (in the form of its hydrochloride) has been shown to induce a rather rare spectrum of tumors in the spleen of Fischer 344 rats. The dose levels necessary for this carcinogenic activity were in... |
|
|
Biotransformation of hydroxylaminobenzene and aminophenol by Pseudomonas putida 2NP8 cells grown in the presence of 3-nitrophenol.
Appl. Environ. Microbiol. 66(6) , 2336-42, (2000) Biotransformation products of hydroxylaminobenzene and aminophenol produced by 3-nitrophenol-grown cells of Pseudomonas putida 2NP8, a strain grown on 2- and 3-nitrophenol, were characterized. Ammonia... |
| N-Phenylhydroxylamine |
| EINECS 202-875-6 |
 CAS#:98-95-3
CAS#:98-95-3 CAS#:586-96-9
CAS#:586-96-9 CAS#:3585-93-1
CAS#:3585-93-1 CAS#:109-77-3
CAS#:109-77-3 CAS#:668418-98-2
CAS#:668418-98-2 CAS#:19865-55-5
CAS#:19865-55-5 CAS#:105-56-6
CAS#:105-56-6 CAS#:27991-08-8
CAS#:27991-08-8 CAS#:111750-22-2
CAS#:111750-22-2 CAS#:1208-86-2
CAS#:1208-86-2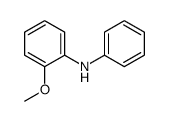 CAS#:1207-92-7
CAS#:1207-92-7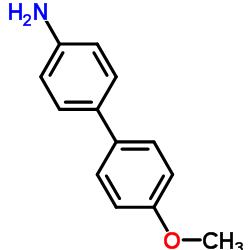 CAS#:1137-77-5
CAS#:1137-77-5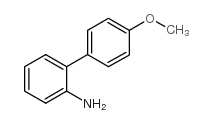 CAS#:38089-03-1
CAS#:38089-03-1![[1,1'-biphenyl]-2-amine,N-phenyl structure](https://image.chemsrc.com/caspic/390/35887-50-4.png) CAS#:35887-50-4
CAS#:35887-50-4![1,1':2',1''-Terphenyl]-4-amine structure](https://image.chemsrc.com/caspic/175/5728-65-4.png) CAS#:5728-65-4
CAS#:5728-65-4 CAS#:76129-23-2
CAS#:76129-23-2 CAS#:32228-99-2
CAS#:32228-99-2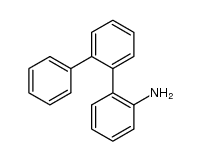 CAS#:56970-23-1
CAS#:56970-23-1
