| Description |
MDL-29951 is a novel glycine antagonist of NMDA receptor activation, with Ki of 0.14 μM for [3H]glycine binding in vitro and in vivo.
|
| Related Catalog |
|
| Target |
Ki: 0.14 μM
|
| In Vitro |
MDL 100,748 and MDL 29,951 are approximately 2000-fold selective for the glycine binding site relative to the glutamate recognition sites[1]. MDL-29951 is found to inhibit the human F16Bpase under these conditions (IC50=2.5 μM). MDL-29951 inhibits the human liver (IC50=2.5 μM), porcine kidney (IC50=1.0 μM), and rabbit liver (IC50=0.21 μM) isoforms of the enzyme, but is significantly less potent against the rat liver isoform (IC50=11 μM)[2]. The MDL29951-activated receptor exhibits other activities associated with GPCR-mediated signaling, including G protein-dependent activation of extracellular signal-regulated kinase 1 and 2 (ERK1/2) and recruitment of β-arrestin. As with recombinant cell systems, MDL29951 promotes Ca2+ signaling responses and inhibition of cyclic adenosine monophosphate (cAMP) accumulation in rat oligodendrocyte precursor cells during the period of peak GPR17 abundance. Effects of MDL29951 are markedly reduced in cells with low GPR17 abundance and are blocked by pranlukast[3].
|
| Kinase Assay |
[3H]JCPP (30.7 Ci/mmol) binding assays are conducted in minivials, incubated for 15 mm at 25°C in 1 mL of 50 mM Tris-HC1 (pH 7.4) containing 10 nM [3H]JCPP, 200 g of membrane protein and unlabeled ligands as indicated. Nonspecific binding is defined using 1 mM L-glutamate. Bound ligand is separated by centrifugation. Specific binding accounted for approximately 80% of total binding.
|
| References |
[1]. Baron BM, et al. Potent indole- and quinoline-containing N-methyl-D-aspartate antagonists acting at the strychnine-insensitive glycine binding site. J Pharmacol Exp Ther. 1992 Sep;262(3):947-56. [2]. Wright SW, et al. 3-(2-carboxyethyl)-4,6-dichloro-1H-indole-2-carboxylic acid: an allosteric inhibitor of fructose-1,6-bisphosphatase at the AMP site. Bioorg Med Chem Lett. 2003 Jun 16;13(12):2055-8. [3]. Harden TK. Enigmatic GPCR finds a stimulating drug. Sci Signal. 2013 Oct 22;6(298):pe34.
|
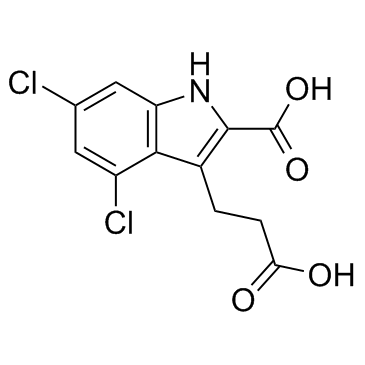
 CAS#:130829-27-5
CAS#:130829-27-5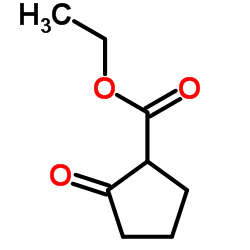 CAS#:611-10-9
CAS#:611-10-9 CAS#:626-43-7
CAS#:626-43-7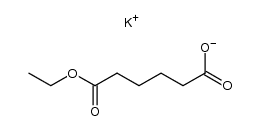 CAS#:24356-36-3
CAS#:24356-36-3