Lomustine
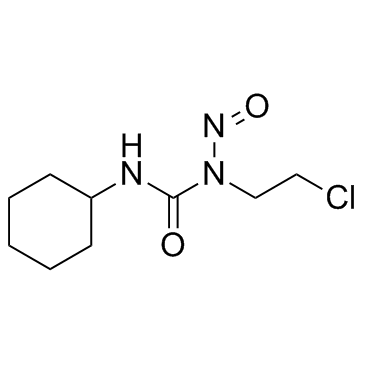
Lomustine structure
|
Common Name | Lomustine | ||
|---|---|---|---|---|
| CAS Number | 13010-47-4 | Molecular Weight | 233.695 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C9H16ClN3O2 | Melting Point | 88-90 | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |


GHS06, GHS08 |
Signal Word | Danger | |
Use of LomustineLomustine is a DNA alkylating agent, with antitumor activity. |
| Name | lomustine |
|---|---|
| Synonym | More Synonyms |
| Description | Lomustine is a DNA alkylating agent, with antitumor activity. |
|---|---|
| Related Catalog | |
| Target |
DNA Alkylator[1] |
| In Vitro | Lomustine is a DNA alkylating agent. Lomustine (CCNU, 0-250 μM) is cytotoxic to the U87-MG cells expressing tumor-derived mutant IDH1, and has little effect on the expression of wild-type IDH1. The combination of Lomustine and procarbazine or vincristine has no additive effect on the killing of cells expressing mutant or wild-type IDH1. Moreover, overexpression of either ALKBH2 or ALKBH3 partially reduces the death HT1080 cells exposed to Lomustine[1]. Lomustine suppresses U87-MG growth with an ED50 of 68.1 μM. Lomustine (30, 40 μM) in combination with docosahexaenoic acid (DHA) darmatically inhibits 2 additional human-derived glioblastoma cell lines, and induces U87-MG apoptosis and necrosis. Lomustine (30 μM) causes G2/M arrest[2]. Lomustine reduces the viability of F98 rat orthotopic glioma cells and Tu-2449 mouse glioma cell line, with IC50s of 20.8 µM and 18.6 µM, respectively[3]. |
| In Vivo | Lomustine (30 mg/kg) in combination with Toca 511 + 5-FC prolongs survival in rats bearing F98 tumor cells. Lomustine (30 mg/kg) combined with Toca-511 + 5-FC also exhibits antitumor activity in the B6C3F1 mice bearing Tu-2449 glioma cells[3]. |
| Kinase Assay | In brief, the oligo substrate (5'-FAM-TAGACATTGCCATTCTCGATAGGXTCCGGTCAAATCTAGACGAATTCCG, X=1-MedA) is used in the assay. ALKBH2 and ALKBH3 is assayed in 50 mM Hepes K (pH 8.0), 50 μM (NH4)2Fe(SO4)2, 50 μM α-KG, 2 mM ascorbic acid, 10 μM oligo substrate, 3 μM enzyme, 50 μg/mL BSA, and 10 mM MgCl2. The reaction is carried out at 37°C and stopped by heating at 95°C for at least 5 min. The oligo substrate is annealed to a reverse complementary oligo, and subjected to extensive DpnII digestion. The digested DNA is separated on 15% TBE-Urea-PAGE gel, and imaged using a Typhoon Scanner[1]. |
| Cell Assay | Initially, cells (5000 cells/well) are cultured in 96-well flat-bottom plates overnight in complete medium to establish a linear growth rate. Spent medium is replaced with new medium supplemented with 2% FBS and varying treatments (100 μL total volume/well). Ethanol-supplemented cells (< 0.5%) serve as the vehicle control. Cells are maintained at 37°C in 5% CO2 in a humidified atmosphere for 24 hours prior to assessment of cell growth with the WST-1 assay reagent. Medium alone combined with the WST-1 assay reagent establishes nonspecific values that are subtracted from the experimental optical density (OD) readings (OD at 450 nm). Vehicle control OD readings serve as standard proliferative potential normalized to 100%. The proliferation index is calculated by dividing the average OD treatment reading by the average OD vehicle reading and multiplying by 100[2]. |
| Animal Admin | Mice[3] Groups of B6C3F1 mice receive PBS or 5-FC only as a control during the study (n = 8 per group). One group of mice (Lomustine Day 1 + PBS) receive one dose of Lomustine (30 mg/kg) on day 1 and a total of six cycles of PBS (800 μL/day, BID for 4 consecutive days every 10 days). The rest of the mice receive 5-FC (500 mg/kg/dose, IP, BID) for 4 consecutive days, plus Lomustine at day 1 (Lomustine Day 1 + 5-FC) or day 43 (Lomustine Day 43 + 5-FC). Cycles of 4-days on, 10-days off 5-FC or PBS are repeated a total of 6 times. Each experiment is terminated at the end of the last 5-FC treatment. All tissues are collected and saved for histopathology. Toxicity in groups receiving Lomustine is compared to the groups receiving PBS or 5-FC alone or in combination with 5-FC at designated time points[3]. Rats[3] Groups of rats receive PBS or 5-FC only as controls during the study (n = 8 per group). One group of rats (Lomustine Day 1 + PBS) receive one dose of Lomustine (30 mg/kg) at day 1 and a total of six cycles of PBS (8 mL/day, BID). The rest of the rats receive 5-FC (500 mg/kg/dose, IP, BID) for 5 consecutive days, followed by 2 days off drug, plus Lomustine on day 1 (Lomustine Day 1 + 5-FC) or day 22 (Lomustine Day 22 + 5-FC). Cycles of 5-days on, 2-days off 5-FC or PBS are repeated a total of 6 times[3]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Melting Point | 88-90 |
| Molecular Formula | C9H16ClN3O2 |
| Molecular Weight | 233.695 |
| Exact Mass | 233.093109 |
| PSA | 61.77000 |
| LogP | 2.76 |
| Vapour Pressure | 0.00142mmHg at 25°C |
| Index of Refraction | 1.583 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H350 |
| Precautionary Statements | P201-P301 + P310-P308 + P313 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | R45 |
| Safety Phrases | S53-S45 |
| RIDADR | 3249 |
| RTECS | YS4900000 |
| Packaging Group | II |
| Hazard Class | 6.1(a) |
| HS Code | 2924299090 |
| Precursor 10 | |
|---|---|
| DownStream 3 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Expression of O(6)-methylguanine-DNA methyltransferase causes lomustine resistance in canine lymphoma cells.
Can. J. Vet. Res. 79 , 201-9, (2015) The DNA repair protein O (6)-methylguanine-DNA methyltransferase (MGMT) causes resistance to nitrosoureas in various human cancers. In this study, we analyzed the correlation between canine lymphomas ... |
|
|
Preliminary study of lomustine in the treatment of intracranial masses in dogs following localization by imaging techniques.
Semin. Vet. Med. Surg. Small Anim. 5(4) , 241-5, (1990)
|
|
|
The nitrosoureas: carmustine (BCNU) and lomustine (CCNU).
Cancer Treat. Rev. 9(4) , 313-30, (1982)
|
| 1-chloroethyl-3-cyclohexyl-1-nitrosourea |
| MFCD00012392 |
| EINECS 235-859-2 |
| Lomustinum |
| Urea, N-(2-chloroethyl)-N'-cyclohexyl-N-nitroso- |
| CINU |
| CeeNU |
| 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea |
| Lomustine |
| CCNU |
| Cecenu |
| Lomustina |
| Belustine |
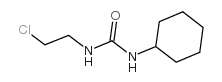 CAS#:13908-11-7
CAS#:13908-11-7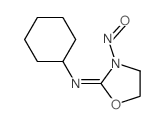 CAS#:76310-06-0
CAS#:76310-06-0 CAS#:80354-49-0
CAS#:80354-49-0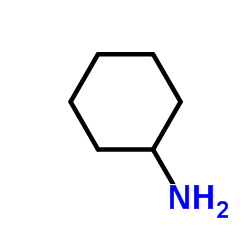 CAS#:108-91-8
CAS#:108-91-8 CAS#:80354-51-4
CAS#:80354-51-4 CAS#:80354-53-6
CAS#:80354-53-6 CAS#:80354-55-8
CAS#:80354-55-8 CAS#:55661-43-3
CAS#:55661-43-3 CAS#:10002-37-6
CAS#:10002-37-6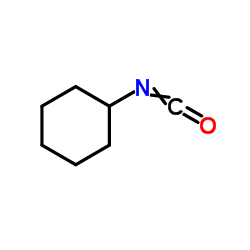 CAS#:3173-53-3
CAS#:3173-53-3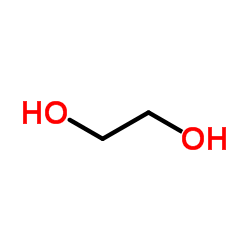 CAS#:107-21-1
CAS#:107-21-1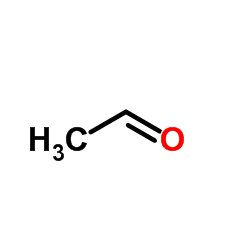 CAS#:75-07-0
CAS#:75-07-0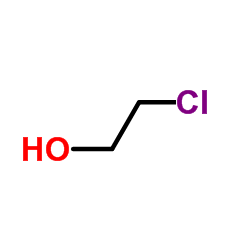 CAS#:107-07-3
CAS#:107-07-3
