Ammonium oxalate
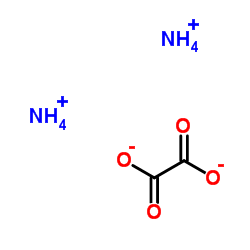
Ammonium oxalate structure
|
Common Name | Ammonium oxalate | ||
|---|---|---|---|---|
| CAS Number | 1113-38-8 | Molecular Weight | 124.096 | |
| Density | N/A | Boiling Point | 365.1ºC at 760 mmHg | |
| Molecular Formula | C2H8N2O4 | Melting Point | 131-135°C | |
| MSDS | N/A | Flash Point | 188.8ºC | |
| Name | Ethanedioic Acid Diammonium Salt |
|---|---|
| Synonym | More Synonyms |
| Boiling Point | 365.1ºC at 760 mmHg |
|---|---|
| Melting Point | 131-135°C |
| Molecular Formula | C2H8N2O4 |
| Molecular Weight | 124.096 |
| Flash Point | 188.8ºC |
| Exact Mass | 124.048409 |
| PSA | 81.08000 |
| Vapour Pressure | 2.51E-06mmHg at 25°C |
| Water Solubility | Soluble |
Synonym:Diammonium oxalate, monohydrate; Ethanedioic acid, diammonium salt monohydrate; Oxalic acid, diammonium salt monohydrate Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
Risk Phrases: 21/22 Section 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW
Harmful in contact with skin and if swallowed. Potential Health Effects Eye: May cause eye irritation. May result in corneal injury. Skin: Harmful if absorbed through the skin. Oxalate is an irritant and may cause dermatitis. Skin lesions begin with epithelial cracking and the formation of slow-healing ulcers. The fingers may appear cyanotic. Ingestion: Ulcerations of the mouth, vomiting of blood, and rapid appearance of shock, convulsions, twitching, tetany, and cardiovascular collapse may occur following ingestion of oxalic acid or its soluble salts. Systemic effects may be due to formation of calcium oxalate which is insoluble at physiological pH and can be deposited in the brain and kidney tubules. Resultant hypocalcemia might disturb the function of the heart and nerves. Mean lethal dose for oxalates in adults is estimated at 10 - 30 grams (143 - 428 mg/kg). Inhalation: Inhalation of oxalic acid produces irritation of the respiratory tract, ulceration of the mucous membranes, headaches, nervousness, cough, vomiting, emaciation, back pain (due to kidney injury), and weakness. Chronic: Inhalation of oxalic acid dust or mist over a long period of time might result in weight loss and respiratory tract inflammation. Rats administered oxalic acid at 2.5 and 5% in the diet for 70 days developed depressed thyroid function and weight loss. A study of railroad car cleaners in Norway who were heavily exposed to oxalic acid solutions and vapors revealed a 53% prevalence of urolithiasis (the formation of urinary stones), compared to a rate of 12% among unexposed workers from the same company. Section 4 - FIRST AID MEASURES Eyes: In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical aid immediately. Skin: In case of contact, immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid immediately. Wash clothing before reuse. Ingestion: If swallowed, do NOT induce vomiting. Get medical aid immediately. If victim is fully conscious, give a cupful of water. Never give anything by mouth to an unconscious person. Inhalation: If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Notes to Physician: Treat symptomatically and supportively. Antidote: Intravenous administration of calcium gluconate or calcium chloride may be required if hypocalcemia or hypocalcemic tetany occur. Section 5 - FIRE FIGHTING MEASURES General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. Extinguishing Media: Use water spray, dry chemical, carbon dioxide, or appropriate foam. Section 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8. Spills/Leaks: Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions. Provide ventilation. Section 7 - HANDLING and STORAGE Handling: Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Minimize dust generation and accumulation. Do not get in eyes, on skin, or on clothing. Keep container tightly closed. Do not ingest or inhale. Discard contaminated shoes. Use only with adequate ventilation. Storage: Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Oxalates slowly corrode steel. Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls: Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low. Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166. Skin: Wear appropriate protective gloves to prevent skin exposure. Clothing: Wear appropriate protective clothing to prevent skin exposure. Respirators: Follow the OSHA respirator regulations found in 29CFR 1910.134 or European Standard EN 149. Always use a NIOSH or European Standard EN 149 approved respirator when necessary. Section 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Solid Appearance: white Odor: odorless pH: 6.4 @ 0.1M Vapor Pressure: Not applicable. Viscosity: Not available. Boiling Point: Decomposes. Freezing/Melting Point: 70 deg C (dec) Autoignition Temperature: Not applicable. Flash Point: Not applicable. Explosion Limits, lower: Not available. Explosion Limits, upper: Not available. Decomposition Temperature: 70 deg C Solubility in water: Soluble. Specific Gravity/Density: 1.5 Molecular Formula: C2H8N2O4.H2O Molecular Weight: 142.11 Section 10 - STABILITY AND REACTIVITY Chemical Stability: Stable under normal temperatures and pressures. Conditions to Avoid: Dust generation, excess heat, Oxalates slowly corrode steel.. Incompatibilities with Other Materials: Strong oxidizing agents. Hazardous Decomposition Products: Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, formic acid, ammonia. Hazardous Polymerization: Has not been reported. Section 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 1113-38-8: RO2750000 CAS# 6009-70-7 unlisted. LD50/LC50: Not available. Not available. Carcinogenicity: Ammonium oxalate anhydrous - Not listed by ACGIH, IARC, NIOSH, NTP, or OSHA. Ammonium oxalate, monohydrate - Not listed by ACGIH, IARC, NIOSH, NTP, or OSHA. Other: See actual entry in RTECS for complete information. Section 12 - ECOLOGICAL INFORMATION Section 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations. Section 14 - TRANSPORT INFORMATION IATA Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.* Hazard Class: 6.1 UN Number: 2811 Packing Group: III IMO Shipping Name: TOXIC SOLID, ORGANIC, N.O.S. Hazard Class: 6.1 UN Number: 2811 Packing Group: III RID/ADR Shipping Name: TOXIC SOLID, ORGANIC, N.O.S. Dangerous Goods Code: 6.1(25C) UN Number: 2811 Section 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives Hazard Symbols: XN Risk Phrases: R 21/22 Harmful in contact with skin and if swallowed. Safety Phrases: S 24/25 Avoid contact with skin and eyes. WGK (Water Danger/Protection) CAS# 1113-38-8: No information available. CAS# 6009-70-7: 1 United Kingdom Occupational Exposure Limits Canada CAS# 1113-38-8 is listed on Canada's DSL List. CAS# 1113-38-8 is not listed on Canada's Ingredient Disclosure List. CAS# 6009-70-7 is not listed on Canada's Ingredient Disclosure List. Exposure Limits US FEDERAL TSCA CAS# 1113-38-8 is listed on the TSCA inventory. CAS# 6009-70-7 is not on the TSCA Inventory. It is a hydrate and exempt from TSCA Inventory requirements (40CFR720.3(u)(2)). SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
|
| Hazard Codes | Xn:Harmful; |
|---|---|
| Risk Phrases | R21/22 |
| Safety Phrases | S24/25 |
| RIDADR | UN 1759 8/PG 3 |
| WGK Germany | 1 |
| RTECS | RO2750000 |
| HS Code | 2917119000 |
|
~% 
Ammonium oxalate CAS#:1113-38-8 |
| Literature: Russian Journal of Applied Chemistry, , vol. 81, # 9 p. 1483 - 1486 |
|
~% 
Ammonium oxalate CAS#:1113-38-8 |
| Literature: Journal fuer Praktische Chemie (Leipzig), , vol. <2>113, p. 92 American Chemical Journal, , vol. 5, p. 198 |
|
~% 
Ammonium oxalate CAS#:1113-38-8 |
| Literature: Zeitschrift fuer Chemie, , p. 302 |
|
~% 
Ammonium oxalate CAS#:1113-38-8 |
| Literature: Journal fuer Praktische Chemie (Leipzig), , vol. <2>113, p. 92 |
|
~% 
Ammonium oxalate CAS#:1113-38-8 |
| Literature: Journal fuer Praktische Chemie (Leipzig), , vol. <2>106, p. 115,136 |
|
~% 
Ammonium oxalate CAS#:1113-38-8 |
| Literature: Chemische Berichte, , vol. 47, p. 1810 Atti della Accademia Nazionale dei Lincei, Classe di Scienze Fisiche, Matematiche e Naturali, Rendiconti, , vol. <5> 23 I, p. 862 |
|
~% 
Ammonium oxalate CAS#:1113-38-8 |
| Literature: Anales de la Real Sociedad Espanola de Fisica y Quimica, , vol. 14, p. 303 |
|
~%
Detail
|
| Literature: Chemische Berichte, , vol. 21, p. 3003 |
|
~%
Detail
|
| Literature: Chemische Berichte, , vol. 47, p. 1810 Atti della Accademia Nazionale dei Lincei, Classe di Scienze Fisiche, Matematiche e Naturali, Rendiconti, , vol. <5> 23 I, p. 862 Atti della Accademia Nazionale dei Lincei, Classe di Scienze Fisiche, Matematiche e Naturali, Rendiconti, , vol. <5> 23 I, p. 860 |
| Precursor 10 | |
|---|---|
| DownStream 9 | |
| HS Code | 2923900090 |
|---|---|
| Summary | 2923900090 other quaternary ammonium salts and hydroxides。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |
| MFCD00013308 |
| EINECS 214-202-3 |
| Ammonium oxalate |
| diazanium,oxalate |

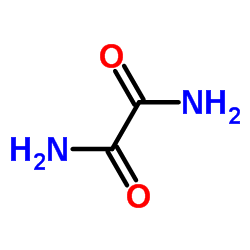


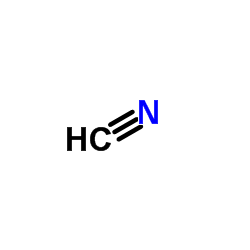
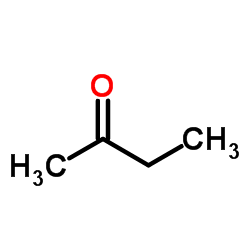
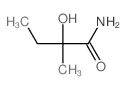
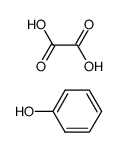

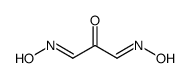

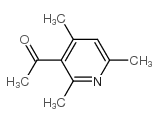 CAS#:56704-25-7
CAS#:56704-25-7 CAS#:471-47-6
CAS#:471-47-6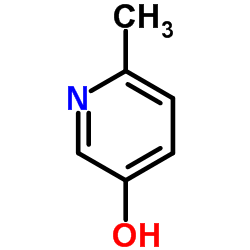 CAS#:1121-78-4
CAS#:1121-78-4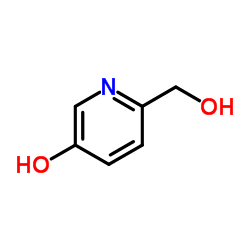 CAS#:40222-77-3
CAS#:40222-77-3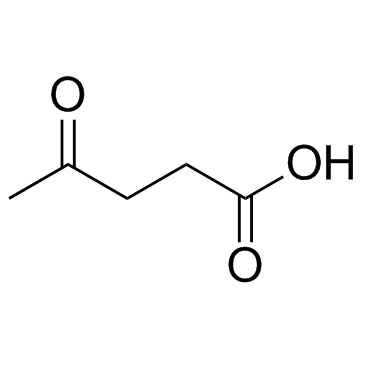 CAS#:123-76-2
CAS#:123-76-2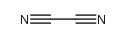 CAS#:460-19-5
CAS#:460-19-5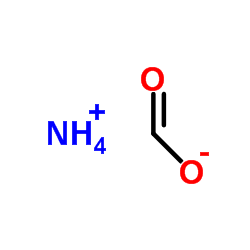 CAS#:540-69-2
CAS#:540-69-2 CAS#:13268-42-3
CAS#:13268-42-3
