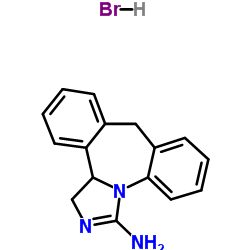
Epinastin HCl
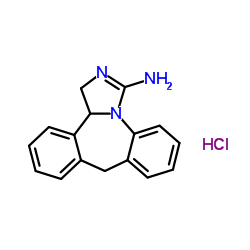
Epinastin HCl structure
|
Common Name | Epinastin HCl | ||
|---|---|---|---|---|
| CAS Number | 108929-04-0 | Molecular Weight | 285.771 | |
| Density | 1.32g/cm3 | Boiling Point | 428ºC at 760 mmHg | |
| Molecular Formula | C16H16ClN3 | Melting Point | >270ºC | |
| MSDS | Chinese USA | Flash Point | 212.7ºC | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of Epinastin HClEpinastine hydrochloride (WAL801 hydrochloride) is an antihistamine and mast cell stabilizer. Epinastine hydrochloride is a potent, selective and orally-active histamine H1 receptor antagonist. Epinastine hydrochloride also inhibits IL-8 release and has an antiallergic action[1][2][3]. |
| Name | Epinastine Hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Epinastine hydrochloride (WAL801 hydrochloride) is an antihistamine and mast cell stabilizer. Epinastine hydrochloride is a potent, selective and orally-active histamine H1 receptor antagonist. Epinastine hydrochloride also inhibits IL-8 release and has an antiallergic action[1][2][3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.32g/cm3 |
|---|---|
| Boiling Point | 428ºC at 760 mmHg |
| Melting Point | >270ºC |
| Molecular Formula | C16H16ClN3 |
| Molecular Weight | 285.771 |
| Flash Point | 212.7ºC |
| Exact Mass | 285.103271 |
| PSA | 41.62000 |
| LogP | 3.46970 |
| Vapour Pressure | 1.56E-07mmHg at 25°C |
| Storage condition | 2-8°C |
| Water Solubility | H2O: soluble38mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | R25 |
| Safety Phrases | 45 |
| RIDADR | UN 2811 6 |
| RTECS | HO4360000 |
| HS Code | 2933990090 |
|
~90% 
Epinastin HCl CAS#:108929-04-0 |
| Literature: USV LIMITED Patent: WO2009/63504 A2, 2009 ; Location in patent: Page/Page column 25 ; |
|
~% 
Epinastin HCl CAS#:108929-04-0 |
| Literature: Arzneimittel-Forschung/Drug Research, , vol. 40, # 4 p. 440 - 446 |
|
~% 
Epinastin HCl CAS#:108929-04-0 |
| Literature: Arzneimittel-Forschung/Drug Research, , vol. 40, # 4 p. 440 - 446 |
|
~% 
Epinastin HCl CAS#:108929-04-0 |
| Literature: Arzneimittel-Forschung/Drug Research, , vol. 40, # 4 p. 440 - 446 |
|
~% 
Epinastin HCl CAS#:108929-04-0 |
| Literature: Arzneimittel-Forschung/Drug Research, , vol. 40, # 4 p. 440 - 446 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Development and validation of an ultra-performance liquid chromatography method for simultaneous analysis of 20 antihistaminics in dietary supplements.
Biomed. Chromatogr. 29(3) , 465-74, (2015) The purpose of this study was to develop and validate an ultra-performance liquid chromatography method for simultaneous analysis of 20 antihistamines (illegal additives) in dietary supplements. The l... |
|
|
Honey bee dopamine and octopamine receptors linked to intracellular calcium signaling have a close phylogenetic and pharmacological relationship.
PLoS ONE 6(11) , e26809, (2011) Three dopamine receptor genes have been identified that are highly conserved among arthropod species. One of these genes, referred to in honey bees as Amdop2, shows a close phylogenetic relationship t... |
|
|
Differential effects of octopamine and tyramine on the central pattern generator for Manduca flight.
J. Comp. Physiol. A. Neuroethol. Sens. Neural. Behav. Physiol. 195(3) , 265-77, (2009) The biogenic amine, octopamine, modulates a variety of aspects of insect motor behavior, including direct action on the flight central pattern generator. A number of recent studies demonstrate that ty... |
| 9,13b-Dihydro-1H-dibenzo[c,f]imidazo[1,5-a]azepin-3-aminhydrochlorid |
| UNII:GFM415S5XL |
| 9,13b-dihydro-1H-dibenzo[c,f]imidazo[1,5-a]azépin-3-amine chlorhydrate |
| 9,13b-Dihydro-1H-dibenz[cf]imidazo[1,5-a]azepine hydrochloride,WAL-801Cl |
| 1H-Dibenz[c,f]imidazo[1,5-a]azepin-3-amine, 9,13b-dihydro-, hydrochloride (1:1) |
| 3-Amino-9,13b-dihydro-1H-dibenzo[c,f]imidazo[1,5-a]azepine Hydrochloride |
| EPINASTINHCL |
| Epinastine hydrochloride |
| Epinastinehydrochloride |
| 1H-Dibenz[c,f]imidazo[1,5-a]azepin-3-amine,9,13b-dihydro-,hydrochloride |
| T B5 G6&6 BN DN CUT&T&J CZ &&HCl |
| EPIMEDIUM P.E |
| EPINASTINE HCL |
| Epinastine |
| 9,13b-dihydro-1H-dibenzo[c,f]imidazo[1,5-a]azepin-3-amine hydrochloride |
| 1H-DIBENZ[C,F]IMIDAZO[1,5-A]AZEPIN-3-AMINE,9,13B-DIHYDRO-, HYDROCHLORIDE (1:1) |
| 9,13b-Dihydro-1H-dibenzo[c,f]imidazo[1,5-a]azepin-3-amine hydrochloride (1:1) |
| Epinastin HCl |
![11H-Dibenzo[b,e]azepine-6-carbonitrile structure](https://image.chemsrc.com/caspic/309/80012-69-7.png)