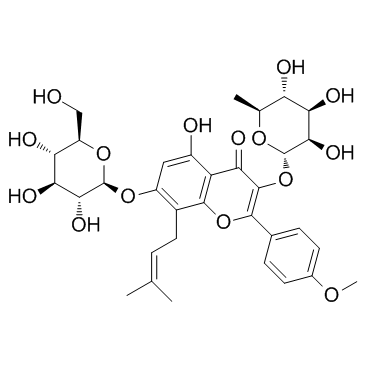
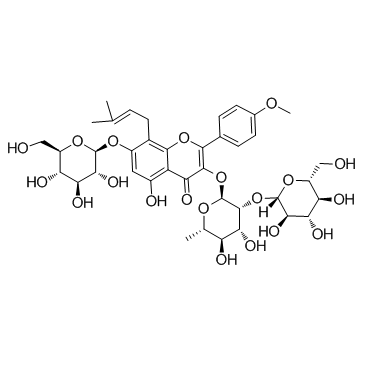
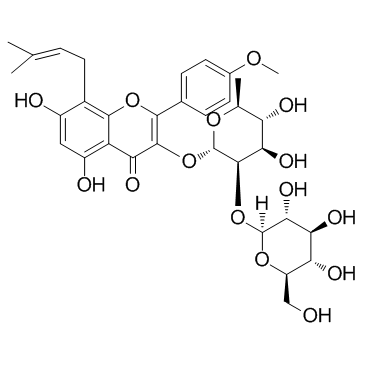
118525-40-9
| Name | 3,5,7-trihydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-enyl)chromen-4-one |
|---|---|
| Synonyms |
3,5,7-Trihydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-4H-chromen-4-one
Icaritin 3,5,7-Trihydroxy-2-(4-methoxyphenyl)-8-(3-methyl-2-buten-1-yl)-4H-chromen-4-one BIDD:ER0021 3,7-dihydroxy-8-prenyl-4'-methoxychrysin Anhydroicaritin |
| Description | Icaritin(Anhydroicaritin) is a component of Epimedium flavonoid isolated from Herba Epimedii; enhances osteoblastic differentiation of mesenchymal stem cells (MSCs) while it inhibits adipogenic differentiation of MSCs by inhibiting PPAR-g pathway.IC50 value:Target: in vitro: Icaritin was unable to promote proliferation, migration and tube like structure formation by human umbilical vein endothelial cells (HUVECs) in vitro [1]. Icaritin potently inhibited proliferation of K562 cells (IC50 was 8 μM) and primary CML cells (IC50 was 13.4 μM for CML-CP and 18 μM for CML-BC), induced CML cells apoptosis and promoted the erythroid differentiation of K562 cells with time-dependent manner. Furthermore, Icaritin was able to suppress the growth of primary CD34+ leukemia cells (CML) and Imatinib-resistant cells, and to induce apoptosis [2]. icaritin strongly inhibited the growth of breast cancer MDA-MB-453 and MCF7 cells. At concentrations of 2-3 μM, icaritin induced cell cycle arrest at the G(2)/M phase accompanied by a down-regulation of the expression levels of the G(2)/M regulatory proteins such as cyclinB, cdc2 and cdc25C. Icaritin at concentrations of 4-5 μM, however, induced apoptotic cell death characterized by the accumulation of the annexin V- and propidium iodide-positive cells, cleavage of poly ADP-ribose polymerase (PARP) and down-regulation of the Bcl-2 expression [3].in vivo: In mouse leukemia model, Icaritin could prolong lifespan of NOD-SCID nude mice inoculated with K562 cells as effective as Imatinib without suppression of bone marrow. Icaritin could up-regulate phospho-JNK or phospho-C-Jun and down-regulate phospho-ERK, phospho-P-38, Jak-2, phospho-Stat3 and phospho-Akt expression with dose- or time-dependent manner [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 582.0±50.0 °C at 760 mmHg |
| Melting Point | 239ºC |
| Molecular Formula | C21H20O6 |
| Molecular Weight | 368.380 |
| Flash Point | 206.7±23.6 °C |
| Exact Mass | 368.125977 |
| PSA | 100.13000 |
| LogP | 4.84 |
| Appearance | light yellow to dark yellow |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.657 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: soluble5mg/mL, clear (warmed) |
| RIDADR | NONH for all modes of transport |
|---|---|
| RTECS | DJ3100870 |
|
~97% 
118525-40-9 |
| Literature: Dell'Agli, Mario; Galli, Germana V.; Dal Cero, Esther; Belluti, Federica; Matera, Riccardo; Zironi, Elisa; Pagliuca, Giampiero; Bosisio, Enrica Journal of Natural Products, 2008 , vol. 71, # 9 p. 1513 - 1517 |
|
~% 
118525-40-9 |
| Literature: Heterocycles, , vol. 26, # 4 p. 935 - 938 |
|
~% 
118525-40-9 |
| Literature: Heterocycles, , vol. 26, # 4 p. 935 - 938 |
| Precursor 3 | |
|---|---|
| DownStream 1 | |