1372540-25-4
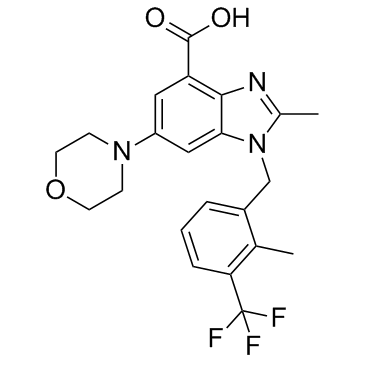
| Name | 2-methyl-1-[[2-methyl-3-(trifluoromethyl)phenyl]methyl]-6-morpholin-4-ylbenzimidazole-4-carboxylic acid |
|---|---|
| Synonyms |
GSK2636771
UNII-DW94IAT0LS cc-414 CS-0747 2-methyl-1-{[2-methyl-3-(trifluoromethyl)phenyl]methyl}-6-(4-morpholinyl)-1H-benzimidazole-4-carboxylic acid QCR-187 2-Methyl-1-[2-methyl-3-(trifluoromethyl)benzyl]-6-(4-morpholinyl)-1H-benzimidazole-4-carboxylic acid GSK-2636771 |
| Description | GSK2636771 is a potent, selective and oral inhibitor of PI3Kβ with a Ki of 0.89 nM and an IC50 of 5.2 nM, showing 900-fold selectivity over p110α and p110γ, and 10-fold selectivity over p110δ isoforms. |
|---|---|
| Related Catalog | |
| Target |
p110β |
| In Vitro | GSK2636771 treatment causes cell viability significantly more decreased in the control cells (p110β-reliant PTEN-deficient PC3 prostate and BT549 and HCC70 breast cancer cell lines) than in PTEN-mutant and PTEN wild-type EEC cells. Inhibition of p110β by GSK2636771 or AZD6482 leads to a marked decrease of AKT phosphorylation only in the control prostate and breast cancer cell lines, whereas only marginal effects on AKT activation are observed in EEC cells[1]. |
| In Vivo | GSK2636771 is a p110β inhibitor, and the p110β primes cells for response to growth factor stimulation. While p110β inhibition suppresses cell and tumor growth, dual targeting of p110α/β enhances apoptosis and provides sustained tumor response in mice model[2]. |
| Cell Assay | Cells are plated in 96-well microtiter plates at densities ranging from 1,500 to 15,000 cells/well, optimized for untreated control cells to be 80-90% confluent at the endpoint of the experiment. After 24 h, cells are treated with serial dilutions (100 pM to 10 µM) of the PI3K pathway inhibitors GDC-0941, A66, TGX-221, GSK2636771, AZD6482, Temsirolimus, AZD8055, PF-04691502, and of the MAPK pathway inhibitors AZD6244, PD0325901, AZD628, and PLX4032. Cell viability is assessed after 72 h of treatment by incubation with CellTiter Blue for 1.5 h. The drug concentration required for survival of 50% of cells relative to untreated cells is determined using GraphPad Prism version 5.0 d. Cell lines that fail to achieve the SF50 to a given drug are nominally assigned as the highest concentration screened (10 µM). At least three independent experiments in triplicate per cell line/targeted drug are performed. Association between a mutation and response to a targeted agent is determined using a Fisher’s exact test, and a two-tailed P value. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 641.3±55.0 °C at 760 mmHg |
| Molecular Formula | C22H22F3N3O3 |
| Molecular Weight | 433.424 |
| Flash Point | 341.7±31.5 °C |
| Exact Mass | 433.161316 |
| PSA | 67.59000 |
| LogP | 3.61 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.606 |
| Hazard Codes | Xi |
|---|