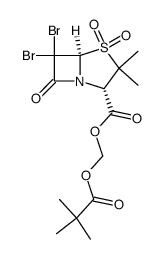
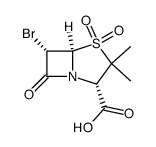
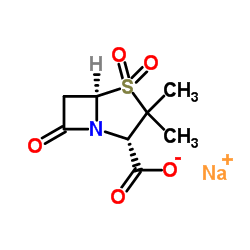
69388-79-0
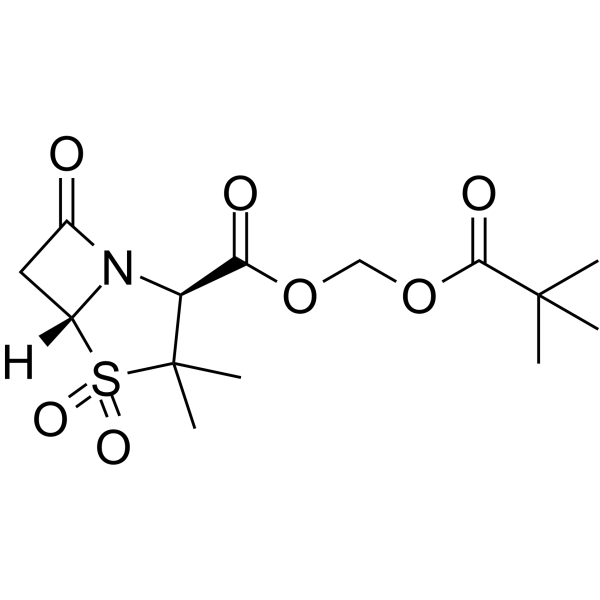
| Name | 2,2-dimethylpropanoyloxymethyl 3,3-dimethyl-4,4,7-trioxo-4λ6-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate |
|---|---|
| Synonyms |
Hydroxymethyl (2S,5R)-3,3-Dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate Pivalate (Ester) 4,4-Dioxide
sulbactam pivaloyl-oxymethyl ester (2S-cis)-3,3-Dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid 4,4-Dioxide (2,2-Dimethyl-1-oxopropoxy)methyl Ester SULBACTAM PIVOXIL Sulbactam pivoxyl 1,1-dioxopenicillanic acid pivaloyloxymethyl ester [(2,2-Dimethylpropanoyl)oxy]methyl (2S,5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate 4,4-dioxide Pivsulbactam Pivoxil sulbactam |
| Description | Sulbactam pivoxil is a prodrug of sulbactam. Sulbactam is a β-lactamase inhibitor which poorly adsorbed from gastrointestinal tract. Sulbactam pivoxil has a better absorption than the parent drug and provides high serum levels after oral administration[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 537.5±50.0 °C at 760 mmHg |
| Molecular Formula | C14H21NO7S |
| Molecular Weight | 347.384 |
| Flash Point | 278.9±30.1 °C |
| Exact Mass | 347.103882 |
| PSA | 115.43000 |
| LogP | -0.02 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.538 |
| Hazard Codes | Xn |
|---|
|
~% 
69388-79-0 |
| Literature: US4714761 A1, ; |
|
~% 
69388-79-0 |
| Literature: US4420426 A1, ; |
|
~% 
69388-79-0 |
| Literature: US4714761 A1, ; |
|
~% 
69388-79-0 |
| Literature: US4528135 A1, ; |
|
~% 
69388-79-0 |
| Literature: US4234579 A1, ; |
|
~82% 
69388-79-0 |
| Literature: Changov, Lubomir S.; Vassileva, Blagina K.; Confino, Maya N.; Agapova, Nely N. Farmaco, 2000 , vol. 55, # 2 p. 134 - 135 |
| Precursor 8 | |
|---|---|
| DownStream 0 | |