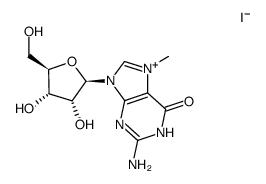
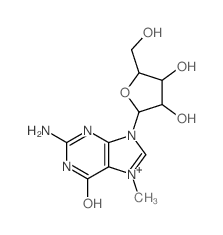
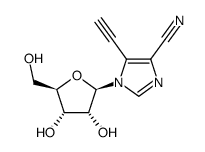
6736-58-9
| Name | Adenosine, 3-deaza |
|---|---|
| Synonyms |
1H-Imidazo[4,5-c]pyridine, 4-amino-1-β-D-ribofuranosyl-
C3-Ado 3-Deaza-D-adenosine 3-DEAZAADENOSINE 1-(β-D-Ribofuranosyl)-1H-imidazo[4,5-c]pyridin-4-amine 1H-Imidazo[4,5-c]pyridin-4-amine, 1-β-D-ribofuranosyl- |
| Description | 3-Deazaadenosine is an inhibitor of S-adenosylhomocysteine hydrolase, with a Ki of 3.9 µM; 3-Deazaadenosine has anti-inflammatory, anti-proliferative and anti-HIV activity. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.15 (HIV-1, A012 isolate), 0.20 µM (HIV-1, A018 isolate)[1] Ki: 3.9 µM (S-adenosylhomocysteine hydrolase)[1] |
| In Vitro | 3-Deazaadenosine is an inhibitor of S-adenosylhomocysteine hydrolase, with a Ki of 3.9 µM. 3-Deazaadenosine shows anti-HIV effect, and inhibits p24 antigen in peripheral blood mononuclear (PBMCs) cells infected with HIV-1 isolates (A012 and A018) with IC50s of 0.15 and 0.20 µM, respectively[1]. 3-Deazaadenosine (1-100 µM) inhibits LPS-induced expression of TNF-α mRNA, increases DNA binding activity of NF-κB, and causes proteolytic degradation of IκBα, but Not IκBβ in RAW 264.7 cells. 3-Deazaadenosine (100 µM) enhances nuclear translocation of NF-κB, but blocks LPS-induced NF-κB transcriptional activity, and such inhibition is augmented by the addition of homocysteine[2]. 3-Deazaadenosine (50, 100 µM) dose-dependently inhibits the phosphorylation of Raf and ERK, protein-dependent kinase 1, protein kinase B (Akt), and forkhead transcription factor FoxO1a. 3-Deazaadenosine (50 µM) suppresses vascular smooth muscle cell (VSMC) proliferation via interfering with Ras signaling[3]. |
| Cell Assay | The HIV-1 strains A012 and A018 are used in the assay. Inhibition of p24 antigen is measured. Briefly, PHA-stimulated peripheral blood mononuclear (PBMCs) are incubated with either HIV-1 strain for 1 h at 37°C at 200-fold the 50% tissue culture infectious dose (TCID50) of the virus stock per 2 × 105 PBMC cells. The TCID50 is defined as the amount of virus stock at which 50% of the inoculated wells are positive. Cells are then grown in microtiter plates with different drug concentrations at 2 × 105 cells per well. On day 4, cells are resuspended and split 1:3 with fresh media and 3-Deazaadenosine. Supernatant p24 antigen is determined on day 7 by ELISA[1]. |
| References |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 665.7±65.0 °C at 760 mmHg |
| Melting Point | 228-229ºC |
| Molecular Formula | C11H14N4O4 |
| Molecular Weight | 266.253 |
| Flash Point | 356.4±34.3 °C |
| Exact Mass | 266.101501 |
| PSA | 126.65000 |
| LogP | 0.18 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.834 |
| Storage condition | 2-8°C |
| Water Solubility | H2O: 10 mg/mL with heating to 60 °C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | 24/25 |
| RIDADR | UN 2811 |
| WGK Germany | 3 |
| Packaging Group | II |
| Hazard Class | 6.1(a) |
| Precursor 8 | |
|---|---|
| DownStream 0 | |
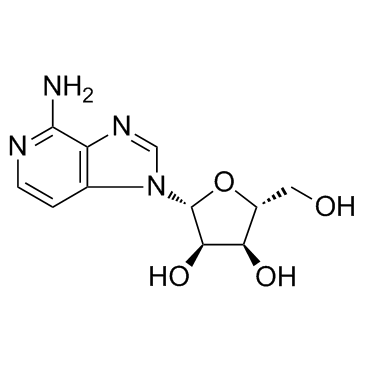
![4-Amino-1-(2’,3’,5’-tri-O-tert-butyldimethylsilyl--D-ribofuranosyl)-imidazo[4,5-a]pyridine structure](https://image.chemsrc.com/caspic/371/147212-86-0.png)
![1H-Imidazo[4,5-c]pyridin-4-amine structure](https://image.chemsrc.com/caspic/318/6811-77-4.png)
![4,7,9-triazabicyclo[4.3.0]nona-2,4,7,10-tetraen-5-amine structure](https://image.chemsrc.com/caspic/182/4261-05-6.png)