482-39-3
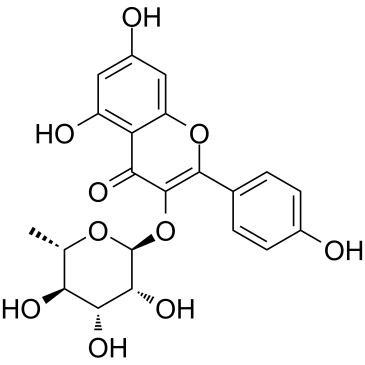
| Name | afzelin |
|---|---|
| Synonyms |
kaempferol 3-O-α-L-rhamnopyranoside
kaempferol-3-rhamnoside 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-3-yl 6-deoxy-α-L-mannopyranoside 5,7-dihydroxy-2-(4-hydroxyphenyl)-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxychromen-4-one Kaempferol-3-O-α-L-rhamnoside Kaempferol 3-O-Alpha-L-Rhamnoside Afzelin Trihydroxy-SL0101 Kaempferol 3-rhamnoside 3-O-α-rhamnosylkaempferol Kaempferin |
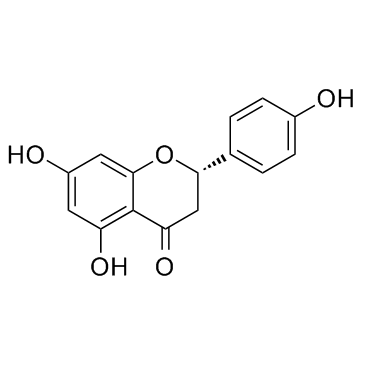
| Description | Afzelin (Kaempferol-3-O-rhamnoside) is is a flavonol glycoside found in Houttuynia cordata Thunberg and is widely used in the preparation of antibacterial and antipyretic agents, detoxicants and for the treatment of inflammation. Afzelin attenuates the mitochondrial damage, enhances mitochondrial biogenesis and decreases the level of mitophagy-related proteins, parkin and PTEN-induced putative kinase 1. Afzelin improves the survival rate and reduces the serum levels of alanine aminotransferase and pro-inflammatory cytokines in D-galactosamine (GalN)/LPS -treated mice[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 765.6±60.0 °C at 760 mmHg |
| Molecular Formula | C21H20O10 |
| Molecular Weight | 432.378 |
| Flash Point | 272.4±26.4 °C |
| Exact Mass | 432.105652 |
| PSA | 170.05000 |
| LogP | 2.37 |
| Vapour Pressure | 0.0±2.7 mmHg at 25°C |
| Index of Refraction | 1.748 |
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
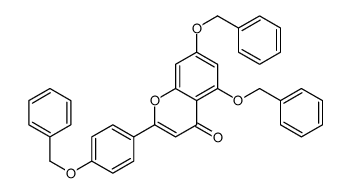
| Precursor 6 | |
|---|---|
| DownStream 2 | |