482-27-9
| Name | isopimpinellin |
|---|---|
| Synonyms |
5,8-Dimethoxypsoralen
4,9-Dimethoxy-furo[3,2-g]chromen-7-one 4,9-Dimethoxyfuro[3,2-g]benzopyran-7-one 4,9-Dimethoxy-7H-furo[3,2-g][1]benzopyran-7-one 4,9-dimethoxyfuro[3,2-g]chromen-7-one 4,9-Dimethoxy-7H-furo[3,2-g]chromen-7-one MFCD00017407 Isopimpinellin |
| Description | Isopimpinellin, an orally active compound isolated from the roots of Pimpinella saxifrage. Isopimpinellin blocks DNA adduct formation and skin tumor initiation by 7,12-dimethylbenz[a]anthracene. Isopimpinellin possesses anti-leishmania effect[1]. |
|---|---|
| Related Catalog | |
| In Vivo | Isopimpinellin (oral gavage, 35-150 mg/kg) inhibits B[a]P-DNA adduct formation and DMBA–DNA adduct formation in SENCAR mice with skin tumor[1]. Animal Model: Female SENCAR mice (7-9 weeks of age) were fed AIN-76A semi-purified diet (Dyets, Bethlehem, PA) for 2 weeks prior to and during the study[1]. Dosage: 35–150 mg/kg. Administration: Oral gavage, suspended in 0.1 mL corn oil at 24 h and 2 h prior to topical treatment with [3H]B[a]P (200 nmol, 1 Ci/mmol) or [3H]DMBA (10 nmol, 10 Ci/mmol) (each in 0.2 mL acetone). Result: Significantly inhibited B[a]P-DNA adduct formation by 37 and 26%, respectively. Isopimpinellin (35, 70 and 150 mg/kg) blocked DMBA–DNA adduct formation by 23, 56 and 69%, respectively |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 448.7±45.0 °C at 760 mmHg |
| Melting Point | 150-151ºC |
| Molecular Formula | C13H10O5 |
| Molecular Weight | 246.215 |
| Flash Point | 225.1±28.7 °C |
| Exact Mass | 246.052826 |
| PSA | 61.81000 |
| LogP | 2.31 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.612 |
| Storage condition | 2-8°C |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300 |
| Precautionary Statements | P264-P301 + P310 |
| Hazard Codes | T+ |
| Risk Phrases | 20/21/22 |
| Safety Phrases | 22-36/37/39-45-36/37-28 |
| RIDADR | UN 2811 6.1 / PGII |
| RTECS | LV1049200 |
|
~80% 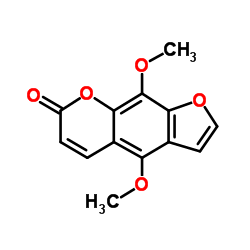
482-27-9 |
| Literature: Bioorganic and Medicinal Chemistry, , vol. 20, # 2 p. 784 - 788 |
|
~% 
482-27-9 |
| Literature: Journal of Organic Chemistry, , vol. 24, p. 523,525 |
|
~% 
482-27-9 |
| Literature: Journal of Organic Chemistry, , vol. 24, p. 523,525 |
|
~% 
482-27-9 |
| Literature: Journal of the Chemical Society, , p. 4163,4168 Chemische Berichte, , vol. 71, p. 344,351 Yakugaku Zasshi, , vol. 58, p. 370,377 Monatshefte fuer Chemie, , vol. 72, p. 179,187 Yakugaku Zasshi, , vol. 60, p. 57,61 Chem.Abstr., , p. 3717 |
|
~% 
482-27-9 |
| Literature: Journal of Organic Chemistry, , vol. 24, p. 523,525 |
|
~% 
482-27-9 |
| Literature: Monatshefte fuer Chemie, , vol. 59, p. 161,170 |
|
~% 
482-27-9 |
| Literature: Journal of the Indian Chemical Society, , vol. 25, p. 139,141 |
| Precursor 7 | |
|---|---|
| DownStream 0 | |