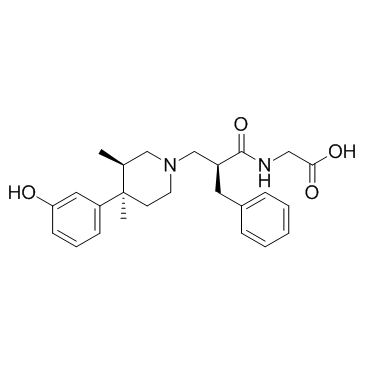
156053-89-3
| Name | Alvimopan |
|---|---|
| Synonyms |
Alvimopan
N-{(2S)-2-benzyl-3-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]propanoyl}glycine N-{(2S)-2-Benzyl-3-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]propanoyl}glycine Unii-Q153V49p3z |
| Description | Alvimopan(LY 246736; ADL 8-2698) is a peripherally acting mu-opioid receptor (PAM-OR, IC50= 1.7 nM) antagonist for accelerating gastrointestinal recovery after surgery. IC50 Value: 1.7 nM (Mu-type opioid receptor) [1]Target: mu-opioid receptorin vitro: The dissociation rate of alvimopan from the micro opioid receptor (t(1/2)=30--44 min) was comparable to that of the long acting partial agonist buprenorphine (t(1/2)=44 min), but was slower than those of the antagonists naloxone (t(1/2)=0.82 min) and N-methylnaltrexone (t(1/2)=0.46 min) [2].in vivo: Alvimopan did not significantly accelerate GI-3 compared with placebo [6 mg: hazard ratio (HR) = 1.20, p = 0.080; 12 mg: HR = 1.24, p = 0.038). However, after adjustment for significant covariates (sex/surgical duration), benefits were significant for both doses (6 mg: HR = 1.24, p = 0.037; 12 mg: HR = 1.26, p = 0.028). Alvimopan also significantly accelerated time to GI-2 (6 mg: HR = 1.37, p = 0.008; 12 mg: HR = 1.33, p = 0.018) and DCO (6 mg: HR = 1.31, p = 0.008; 12 mg: HR = 1.28, p = 0.015) [3]. Alvimopan (1 and 3 mg/kg) significantly reversed this delayed GI transit when administered 45 min prior to surgery. However, the effects of alvimopan were less pronounced when administered following surgery [4].Toxicity:The most common treatment-emergent adverse events across all treatment groups were nausea, vomiting, and hypotension; the incidence of nausea and vomiting was reduced by 53 percent in thealvimopan 12-mg group [5].Clinical trial: Intercostal Nerve Block With Liposome Bupivacaine in Subjects Undergoing Posterolateral Thoracotomy. Phase 3 |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 684.1±55.0 °C at 760 mmHg |
| Melting Point | 210-213ºC |
| Molecular Formula | C25H32N2O4 |
| Molecular Weight | 424.533 |
| Flash Point | 367.5±31.5 °C |
| Exact Mass | 424.236206 |
| PSA | 89.87000 |
| LogP | 3.38 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.572 |
| Storage condition | 2-8°C |
| Symbol |


GHS07, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H400 |
| Precautionary Statements | P273-P280-P305 + P351 + P338-P337 + P313-P391 |
| RIDADR | UN 3077 9 / PGIII |
| HS Code | 2933399090 |
|
~% 
156053-89-3 |
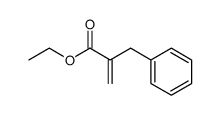
| Literature: Zimmerman, Dennis M.; Gidda, Jaswant S.; Cantrell, Buddy E.; Schoepp, Darryle D.; Johnson, Bryan G.; Leander, J. David Journal of Medicinal Chemistry, 1994 , vol. 37, # 15 p. 2262 - 2265 |
|
~% 
156053-89-3 |
| Literature: Journal of Medicinal Chemistry, , vol. 37, # 15 p. 2262 - 2265 |
|
~% 
156053-89-3 |
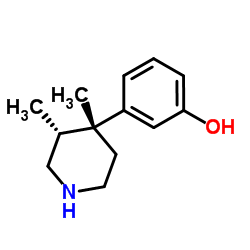
| Literature: Journal of Medicinal Chemistry, , vol. 37, # 15 p. 2262 - 2265 |
|
~% 
156053-89-3 |
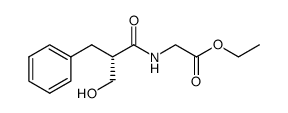
| Literature: WO2011/161646 A2, ; |
|
~% 
156053-89-3 |
| Literature: WO2011/161646 A2, ; |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |