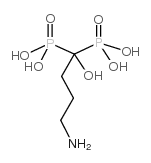
121268-17-5
| Name | alendronate sodium trihydrate |
|---|---|
| Synonyms |
Alendronate sodium
(4-Amino-1-hydroxy-1-phosphonobutyl)phosphonic Acid Monosodium Salt Trihydrate 4-amino-1-hydroxy-1-phosphonobutyl phosphonic acid,monosodium,Alendronate sodium trihydrate Phosphonic acid, (4-amino-1-hydroxybutylidene)bis-, sodium salt, hydrate (1:1:3) Alendronate sodium trihydrate Sodium Alendronate Trihydrate hydrogène (4-amino-1-hydroxy-1-phosphonobutyl)phosphonate de sodium trihydrate alendronate sodium hydrate Monosodium (4-Amino-1-hydroxy-1-phosphonobutyl)phosphonate Trihydrate Alendronate monosodium trihydrate MFCD01748233 Alendronic acid monosodium salt trihydrate Sodium hydrogen (4-amino-1-hydroxy-1-phosphonobutyl)phosphonate hydrate (1:1:3) onclast Adronat Natriumhydrogen-(4-amino-1-hydroxy-1-phosphonobutyl)phosphonattrihydrat 4-amino-1-hydroxy-1-phosphonobutyl phosphonic acid,monosodium MK-217 Dronal Sodium alendronate Alendronate (sodium hydrate) |
| Description | Alendronate (sodium hydrate) is a farnesyl diphosphate synthase inhibitor with IC50 of 460 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 460 nM (farnesyl diphosphate synthase) |
| In Vitro | Alendronate, acting directly on osteoclasts, inhibits a rate-limiting step in the cholesterol biosynthesis pathway, essential for osteoclast function[1]. Alendronate inhibits the isoprenoid biosynthesis pathway and interferes with protein prenylation, as a result of reduced geranylgeranyl diphosphate levels. Alendronate inhibits the incorporation of [3H]mevalonolactone into proteins of 18-25 kDa and into nonsaponifiable lipids, including sterols in osteoclasts[2]. Alendronate causes a dose-dependent inhibition of [3H]MVA incorporation into sterols and a concomitant increase in incorporation of radiolabel into IPP and DMAPP[3]. |
| In Vivo | Alendronate causes erosions in the rabbit stomach, but not antral ulceration in rats. Alendronate increases the incidence and size of indomethacin-induced antral ulcers. Alendronate also enhances indomethacin-induced gastricdamage in the rat, and delays gastric ulcer healing[4]. Alendronate (0.04-0.1 mg/kg twice weekly or 0.1 mg/kg weekly) partially blocks the establishment of bone metastases by human PC-3 ML cells and results in tumor formation in the peritoneum and other soft tissues. Alendronate pretreatment of mice (0.1 mg/kg twice weekly or weekly) and dosing along with taxol (10-50 mg/kg/day, twice weekly, or weekly) blocks the growth of PC-3 ML tumors in the bone marrow and soft tissues in a statistically significant manner and improves survival rates significantly by 4-5 weeks[5]. |
| Kinase Assay | Rat liver cytosol is prepared from a separate piece of liver from rat. Assays are carried out in a total volume of 0.1 mL containing 10 mg of cytosolic protein, and components according to Rilling. All reaction components (except IPP) are mixed and kept on ice for 15 min. Reactions are initiated by the addition of [14C]IPP and incubation at 37°C. Reactions are stopped after 5 min by addition of 0.4 mL MeOH/HCl (4/1, by vol.) and the samples are incubated a further 15 min to hydrolyze the allylic pyrophosphates to petroleum ether-extractable products. Following addition of 0.5 mL water and 1 mL petroleum ether, 50% of the upper (petroleum ether-extractable) phase is taken for liquid scintillation analysis. Preliminary experiments indicated that the reaction is linear with time and protein under these conditions, and no more than 10% of the substrate is consumed. |
| References |
| Density | 1.857g/cm3 |
|---|---|
| Boiling Point | 616.7ºC at 760 mmHg |
| Melting Point | 245°C |
| Molecular Formula | C4H18NNaO10P2 |
| Molecular Weight | 325.124 |
| Flash Point | 326.7ºC |
| Exact Mass | 325.030365 |
| PSA | 211.45000 |
| Storage condition | −20°C |
| Stability | Store in freezer |
| Water Solubility | water, double-distilled: 10 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn,Xi |
| Risk Phrases | R22:Harmful if swallowed. R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . S36:Wear suitable protective clothing . |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | SZ6523500 |
| HS Code | 29310095 |
|
~68% 
121268-17-5 |
| Literature: GENERICS [UK] LIMITED; MERCK DEVELOPMENT CENTRE PRIVATE LIMITED Patent: WO2008/4000 A1, 2008 ; Location in patent: Page/Page column 20 ; |
|
~% 
121268-17-5 |
| Literature: WO2005/44831 A2, ; Page/Page column 11 ; |
|
~92% 
121268-17-5 |
| Literature: Migianu-Griffoni, Evelyne; Chebbi, Imene; Kachbi, Souad; Monteil, Maelle; Sainte-Catherine, Odile; Chaubet, Frederic; Oudar, Olivier; Lecouvey, Marc Bioconjugate Chemistry, 2014 , vol. 25, # 2 p. 224 - 230 |
|
~80% 
121268-17-5 |
| Literature: IPCA Laboratories Limited Patent: EP1803727 A1, 2007 ; Location in patent: Page/Page column 5 ; |
|
~87% 
121268-17-5 |
| Literature: Mizrahi, Dana M.; Waner, Trevor; Segall, Yoffi Phosphorus, Sulfur and Silicon and Related Elements, 2001 , vol. 173, p. 1 - 25 |
| Precursor 4 | |
|---|---|
| DownStream 1 | |