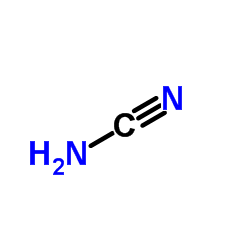
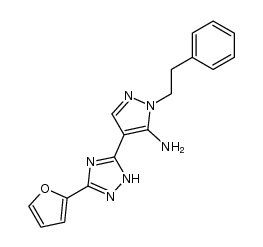
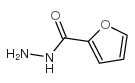
160098-96-4
| Name | 2-(2-furanyl)-7-(2-phenylethyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine |
|---|---|
| Synonyms |
7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine
2-(Furan-2-yl)-7-phenethyl-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine SCH58261 SCH 58261 |
| Description | SCH 58261 is the adenosine A2A receptor competitive antagonist. Displays 323-, 53- and 100-fold selectivity over A1, A2B and A3 receptors, respectively.target: adenosine A2A receptorIC50: 15 nM [3]in vitro: NK cells were cultured in NK cell media and preincubated with or without 1 uM SCH58261 30 min before simulation with indicated concentrations of IL-18 (R & D Systems) and IL-12p70 (Australian Biosearch) in the presence or absence of NECA (1 uM) or CGS-21680 (100 nM).[1]in vivo: it was demonstrated that the selective antagonist of the A2Areceptor, SCH58261, administered i.p. starting from the early minutes after ischemia induction, reduces ischemic brain damage and neurological deficit 24 h thereafter. vehicle-rats received saline with Tween 80 (1 %) administered (i.p.) .SCH58261 (0.01 mg/kg, i.p.), administered twice/day for 7 days [2] |
|---|---|
| Related Catalog | |
| References |
| Density | 1.54g/cm3 |
|---|---|
| Molecular Formula | C18H15N7O |
| Molecular Weight | 345.35800 |
| Exact Mass | 345.13400 |
| PSA | 100.06000 |
| LogP | 3.14010 |
| Index of Refraction | 1.807 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| HS Code | 2934999090 |
|
~20% 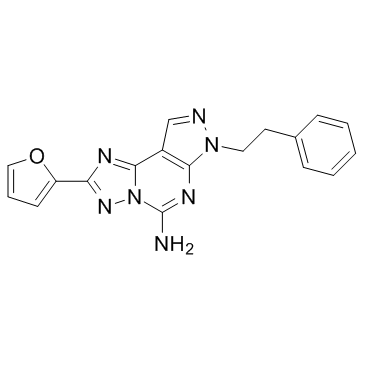
160098-96-4 |
| Literature: Baraldi, Pier Giovanni; Cacciari, Barbara; Spalluto, Giampiero; Pineda De Las Infantas Y Villatoro, Maria Jose; Zocchi, Cristina; Dionisotti, Silvio; Ongini, Ennio Journal of Medicinal Chemistry, 1996 , vol. 39, # 5 p. 1164 - 1171 |
|
~% 
160098-96-4 |
| Literature: Baraldi, Pier Giovanni; Cacciari, Barbara; Spalluto, Giampiero; Pineda De Las Infantas Y Villatoro, Maria Jose; Zocchi, Cristina; Dionisotti, Silvio; Ongini, Ennio Journal of Medicinal Chemistry, 1996 , vol. 39, # 5 p. 1164 - 1171 |
|
~% 
160098-96-4 |
| Literature: Baraldi, Pier Giovanni; Cacciari, Barbara; Spalluto, Giampiero; Pineda De Las Infantas Y Villatoro, Maria Jose; Zocchi, Cristina; Dionisotti, Silvio; Ongini, Ennio Journal of Medicinal Chemistry, 1996 , vol. 39, # 5 p. 1164 - 1171 |
|
~% 
160098-96-4 |
| Literature: Baraldi, Pier Giovanni; Cacciari, Barbara; Spalluto, Giampiero; Pineda De Las Infantas Y Villatoro, Maria Jose; Zocchi, Cristina; Dionisotti, Silvio; Ongini, Ennio Journal of Medicinal Chemistry, 1996 , vol. 39, # 5 p. 1164 - 1171 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |