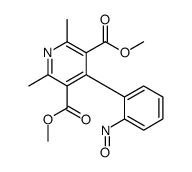
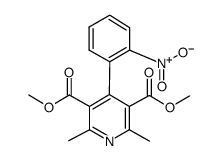
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
US7975000
-
CHEMICAL NAME :
-
3,5-Pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4-(2-nitrophenyl)-, dimethyl ester
-
CAS REGISTRY NUMBER :
-
21829-25-4
-
BEILSTEIN REFERENCE NO. :
-
0497773
-
LAST UPDATED :
-
199806
-
DATA ITEMS CITED :
-
42
-
MOLECULAR FORMULA :
-
C17-H18-N2-O6
-
MOLECULAR WEIGHT :
-
346.37
-
WISWESSER LINE NOTATION :
-
T6M DHJ B1 CVO1 DR BNW& EVO1 F1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
34286 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Gastrointestinal - nausea or vomiting Gastrointestinal - necrotic changes
-
REFERENCE :
-
JTCTDW Journal of Toxicology, Clinical Toxicology. (Marcel Dekker, 270 Madison Ave., New York, NY 10016) V.19- 1982- Volume(issue)/page/year: 33,725,1995
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
105 mg/kg/26W-I
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Olfaction) - effect, not otherwise specified
-
REFERENCE :
-
LANCAO Lancet. (7 Adam St., London WC2N 6AD, UK) V.1- 1823- Volume(issue)/page/year: 339,1382,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
2400 ug/kg/3D-I
-
TOXIC EFFECTS :
-
Behavioral - toxic psychosis
-
REFERENCE :
-
BMJOAE British Medical Journal. (British Medical Assoc., BMA House, Tavistock Sq., London WC1H 9JR, UK) V.1- 1857- Volume(issue)/page/year: 304,1225,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
11200 ug/kg
-
TOXIC EFFECTS :
-
Vascular - BP lowering not characterized in autonomic section Lungs, Thorax, or Respiration - respiratory depression
-
REFERENCE :
-
JJTOEX Japanese Journal of Toxicology. (Yakugyo Jihosha, Hokushin Bldg., 2-36 Jinbo-cho, Kanda, Chiyoda-ku, Tokyo, 101, Japan) V.1- 1988- Volume(issue)/page/year: 5,79,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
31 mg/kg/11W-I
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - dyspnea Nutritional and Gross Metabolic - body temperature increase
-
REFERENCE :
-
AHJOA2 American Heart Journal. (C.V. Mosby Co., 11830 Westline Industrial Dr., St. Louis, MO 63146) V.1- 1925- Volume(issue)/page/year: 108,611,1984
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
800 ug/kg/1D-I
-
TOXIC EFFECTS :
-
Brain and Coverings - changes in circulation (hemorrhage, thrombosis, etc.) Behavioral - headache Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
PGMJAO Postgraduate Medical Journal. (Blackwell Scientific Pub. Ltd., POB 88, Oxford, UK) V.1- 1925- Volume(issue)/page/year: 62,1029,1986
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
1143 ug/kg/2D-I
-
TOXIC EFFECTS :
-
Behavioral - hallucinations, distorted perceptions Behavioral - toxic psychosis
-
REFERENCE :
-
AJMEAZ American Journal of Medicine. (Technical Pub., 875 Third Ave., New York, NY 10022) V.1- 1946- Volume(issue)/page/year: 81,705,1986
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
600 ug/kg/45M-I
-
TOXIC EFFECTS :
-
Vascular - BP lowering not characterized in autonomic section Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
AIMDAP Archives of Internal Medicine. (AMA, 535 N. Dearborn St., Chicago, IL 60610) V.1- 1908- Volume(issue)/page/year: 147,556,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
8571 ug/kg
-
TOXIC EFFECTS :
-
Cardiac - change in rate Vascular - BP lowering not characterized in autonomic section
-
REFERENCE :
-
HETOEA Human & Experimental Toxicology. (Macmillan Press Ltd., Brunel Road, Houndmills, Basingstoke, Hampshire, RG21 2XS, UK) V.9- 1990- Volume(issue)/page/year: 9,309,1990
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
143 ug/kg/1D
-
TOXIC EFFECTS :
-
Vascular - BP lowering not characterized in autonomic section
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 22,380,1972
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
70 mg/kg
-
TOXIC EFFECTS :
-
Cardiac - cardiomyopathy including infarction Vascular - BP lowering not characterized in autonomic section Endocrine - hyperglycemia
-
REFERENCE :
-
PEDIAU Pediatrics. (American Academy of Pediatrics, P.O. Box 1034, Evanston, IL 60204) V.1- 1948- Volume(issue)/page/year: 86,91,1990
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
714 ug/kg
-
TOXIC EFFECTS :
-
Cardiac - change in rate Vascular - BP lowering not characterized in autonomic section
-
REFERENCE :
-
AEMED3 Annals of Emergency Medicine. (American College of Emergency Physicians, 1125 Executive Circle, Irving, TX 75038) Volume(issue)/page/year: 22,196,1993
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1022 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - changes in motor activity (specific assay) Lungs, Thorax, or Respiration - dyspnea Lungs, Thorax, or Respiration - respiratory stimulation
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 22,1,1972
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
230 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YKYUA6 Yakkyoku. Pharmacy. (Nanzando, 4-1-11, Yushima, Bunkyo-ku, Tokyo, Japan) V.1- 1950- Volume(issue)/page/year: 28,1451,1977
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>10 gm/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - convulsions or effect on seizure threshold Behavioral - ataxia
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 5,1831,1971
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
6 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
ARTODN Archives of Toxicology. (Springer-Verlag, Heidelberger Pl. 3, D-1000 Berlin 33, Fed. Rep. Ger.) V.32- 1974- Volume(issue)/page/year: 54,275,1983
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
202 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
USXXAM United States Patent Document. (U.S. Patent Office, Box 9, Washington, DC 20231) Volume(issue)/page/year: #3644627
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
185 mg/kg
-
TOXIC EFFECTS :
-
Vascular - BP lowering not characterized in autonomic section Vascular - regional or general arteriolar or venous dilation
-
REFERENCE :
-
PCJOAU Pharmaceutical Chemistry Journal (English Translation). Translation of KHFZAN. (Plenum Pub. Corp., 233 Spring St., New York, NY 10013) No.1- 1967- Volume(issue)/page/year: 16,817,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>10 gm/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - convulsions or effect on seizure threshold Behavioral - ataxia
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 5,1831,1971
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
4200 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - changes in motor activity (specific assay) Lungs, Thorax, or Respiration - dyspnea Lungs, Thorax, or Respiration - respiratory stimulation
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 22,1,1972
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - cat
-
DOSE/DURATION :
-
100 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - changes in motor activity (specific assay) Lungs, Thorax, or Respiration - dyspnea Lungs, Thorax, or Respiration - respiratory stimulation
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 22,1,1972
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - cat
-
DOSE/DURATION :
-
500 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - changes in motor activity (specific assay) Lungs, Thorax, or Respiration - dyspnea Lungs, Thorax, or Respiration - respiratory stimulation
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 22,1,1972
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
504 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - dyspnea Nutritional and Gross Metabolic - body temperature decrease
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 35,915,1985
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
2 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - changes in motor activity (specific assay) Lungs, Thorax, or Respiration - dyspnea Lungs, Thorax, or Respiration - respiratory stimulation
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 22,1,1972 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
4800 mg/kg/4W-I
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Kidney, Ureter, Bladder - urine volume increased Nutritional and Gross Metabolic - changes in potassium
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 5,1831,1971
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
14400 mg/kg/24W-I
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Kidney, Ureter, Bladder - urine volume increased Nutritional and Gross Metabolic - changes in potassium
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 5,1831,1971
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1152 mg/kg/12W-I
-
TOXIC EFFECTS :
-
Cardiac - changes in heart weight Liver - changes in liver weight Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 35,915,1985
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
91250 mg/kg/2Y-C
-
TOXIC EFFECTS :
-
Nutritional and Gross Metabolic - weight loss or decreased weight gain Related to Chronic Data - death
-
REFERENCE :
-
TOXID9 Toxicologist. (Soc. of Toxicology, Inc., 475 Wolf Ledge Parkway, Akron, OH 44311) V.1- 1981- Volume(issue)/page/year: 36(1,pt2),174,1997 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
200 ug/kg
-
SEX/DURATION :
-
female 27 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - other effects Reproductive - Effects on Embryo or Fetus - other effects to embryo
-
REFERENCE :
-
GOBIDS Gynecologic and Obstetric Investigation. (S. Karger Pub., Inc., 79 Fifth Ave., New York, NY 10003) V.9- 1978- Volume(issue)/page/year: 24,151,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
200 ug/kg
-
SEX/DURATION :
-
female 33 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - other effects Reproductive - Effects on Embryo or Fetus - other effects to embryo
-
REFERENCE :
-
BJOGAS British Journal of Obstetrics and Gynaecology. (Blackwell Scientific Pub. Ltd., POB 88, Oxford, UK) V.82- 1975- Volume(issue)/page/year: 100,959,1993
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
550 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Effects on Newborn - stillbirth
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 19,2503,1991
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
10 mg/kg
-
SEX/DURATION :
-
female 21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system
-
REFERENCE :
-
PEREBL Pediatric Research. (Williams & Wilkins Co., 428 E. Preston St., Baltimore, MD 21202) V.1- 1967- Volume(issue)/page/year: 26,442,1989
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
40 mg/kg
-
SEX/DURATION :
-
female 14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
TOLED5 Toxicology Letters. (Elsevier Science Pub. B.V., POB 211, 1000 AE Amsterdam, Netherlands) V.1- 1977- Volume(issue)/page/year: 40,127,1988
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
275 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Effects on Embryo or Fetus - fetal death
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 21(Suppl 4),S1095,1993
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
15 mg/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
REPTED Reproductive Toxicology. (Pergamon Press Inc., Maxwell House, Fairview Park, Elmsford, NY 10523) V.1- 1987- Volume(issue)/page/year: 11,207,1997
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
5 gm/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 21(Suppl 4),S1115,1993
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
33200 ug/kg
-
SEX/DURATION :
-
female 16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
TJADAB Teratology, The International Journal of Abnormal Development. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968- Volume(issue)/page/year: 45,247,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
780 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea) Reproductive - Fertility - other measures of fertility Reproductive - Effects on Newborn - live birth index (measured after birth)
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 21(Suppl 4),S1125,1993
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
6 mg/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - ovaries, fallopian tubes
-
REFERENCE :
-
YHHPAL Yaoxue Xuebao. Acta Pharmaceutica Sinica. Pharmaceutical Journal. (China International Book Trading Corp., POB 2820, Beijing, Peop. Rep. China) V.1- 1953- Suspended 1966-78. Volume(issue)/page/year: 21,740,1986
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
6 mg/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - ovaries, fallopian tubes
-
REFERENCE :
-
YHHPAL Yaoxue Xuebao. Acta Pharmaceutica Sinica. Pharmaceutical Journal. (China International Book Trading Corp., POB 2820, Beijing, Peop. Rep. China) V.1- 1953- Suspended 1966-78. Volume(issue)/page/year: 21,740,1986
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
94 ug/kg/30M-C
-
SEX/DURATION :
-
female 18 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system Reproductive - Maternal Effects - other effects
-
REFERENCE :
-
AJOGAH American Journal of Obstetrics and Gynecology. (C.V. Mosby Co., 11830 Westline Industrial Dr., St. Louis, MO 63146) V.1- 1920- Volume(issue)/page/year: 157,1003,1987 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X6165 No. of Facilities: 26 (estimated) No. of Industries: 1 No. of Occupations: 1 No. of Employees: 766 (estimated) No. of Female Employees: 426 (estimated)
|



![1-(2-nitrophenyl)-N-[(2-nitrophenyl)-[(2-nitrophenyl)methylideneamino]methyl]methanimine structure](https://image.chemsrc.com/caspic/092/126192-88-9.png)


![2-Methyl-4-(2-nitrophenyl)-5-oxo-5,7-dihydrofuro[3,4-b]pyridin-3-carbonsaeure structure](https://image.chemsrc.com/caspic/135/105378-62-9.png)