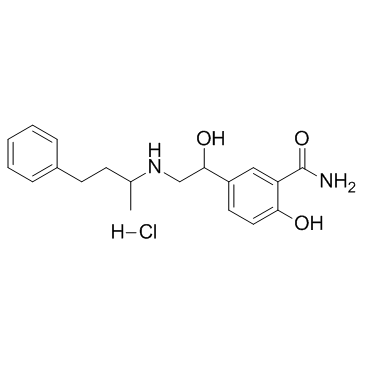
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
CV5376000
-
CHEMICAL NAME :
-
Benzamide, 2-hydroxy-5-(1-hydroxy-2-((1-methyl-3-phenylpropyl)am ino)ethyl)-, monohydrochloride
-
CAS REGISTRY NUMBER :
-
32780-64-6
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
27
-
MOLECULAR FORMULA :
-
C19-H24-N2-O3.Cl-H
-
MOLECULAR WEIGHT :
-
364.91
-
WISWESSER LINE NOTATION :
-
ZVR BQ EYQ1MY1&2R &GH
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
244 mg/kg/76D-I
-
TOXIC EFFECTS :
-
Liver - liver function tests impaired Lungs, Thorax, or Respiration - other changes
-
REFERENCE :
-
AJMEAZ American Journal of Medicine. (Technical Pub., 875 Third Ave., New York, NY 10022) V.1- 1946- Volume(issue)/page/year: 87,235,1989
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
360 mg/kg/60D-I
-
TOXIC EFFECTS :
-
Liver - jaundice (or hyperbilirubinemia) hepatocellular Liver - liver function tests impaired
-
REFERENCE :
-
AIMEAS Annals of Internal Medicine. (American College of Physicians, 4200 Pine St., Philadelphia, PA 19104) V.1- 1927- Volume(issue)/page/year: 113,210,1990
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
1620 mg/kg/27W-I
-
TOXIC EFFECTS :
-
Gastrointestinal - other changes Liver - jaundice (or hyperbilirubinemia) hepatocellular Kidney, Ureter, Bladder - other changes in urine composition
-
REFERENCE :
-
AIMEAS Annals of Internal Medicine. (American College of Physicians, 4200 Pine St., Philadelphia, PA 19104) V.1- 1927- Volume(issue)/page/year: 113,210,1990
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
53 mg/kg/53D-I
-
TOXIC EFFECTS :
-
Liver - jaundice (or hyperbilirubinemia) hepatocellular Skin and Appendages - dermatitis, other (after systemic exposure)
-
REFERENCE :
-
AIMEAS Annals of Internal Medicine. (American College of Physicians, 4200 Pine St., Philadelphia, PA 19104) V.1- 1927- Volume(issue)/page/year: 113,210,1990
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2114 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Behavioral - convulsions or effect on seizure threshold Nutritional and Gross Metabolic - body temperature decrease
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 21,6307,1987
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
107 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold
-
REFERENCE :
-
HOIZAK Hokkaido Igaku Zasshi. Hokkaido Journal of Medical Science. (Hokkaido Daigaku Igakubu, Nishi-7-chome, Kita-15-jo, Kita-ku, Sapporo, Japan) V.1- 1923- Volume(issue)/page/year: 53,15,1978
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
53 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold
-
REFERENCE :
-
HOIZAK Hokkaido Igaku Zasshi. Hokkaido Journal of Medical Science. (Hokkaido Daigaku Igakubu, Nishi-7-chome, Kita-15-jo, Kita-ku, Sapporo, Japan) V.1- 1923- Volume(issue)/page/year: 53,15,1978
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1450 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - convulsions or effect on seizure threshold
-
REFERENCE :
-
HOIZAK Hokkaido Igaku Zasshi. Hokkaido Journal of Medical Science. (Hokkaido Daigaku Igakubu, Nishi-7-chome, Kita-15-jo, Kita-ku, Sapporo, Japan) V.1- 1923- Volume(issue)/page/year: 53,15,1978
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
114 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold
-
REFERENCE :
-
HOIZAK Hokkaido Igaku Zasshi. Hokkaido Journal of Medical Science. (Hokkaido Daigaku Igakubu, Nishi-7-chome, Kita-15-jo, Kita-ku, Sapporo, Japan) V.1- 1923- Volume(issue)/page/year: 53,15,1978
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
47 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold
-
REFERENCE :
-
HOIZAK Hokkaido Igaku Zasshi. Hokkaido Journal of Medical Science. (Hokkaido Daigaku Igakubu, Nishi-7-chome, Kita-15-jo, Kita-ku, Sapporo, Japan) V.1- 1923- Volume(issue)/page/year: 53,15,1978
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
>1500 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - tremor Behavioral - ataxia Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
JZKEDZ Jitchuken Zenrinsho Kenkyuho. Central Institute for Experimental Animals, Research Reports. (Jikken Dobutsu Chuo Kenkyusho, 1433 Nogawa, Takatsu-ku, Kawasaki 211, Japan) V.1- 1975- Volume(issue)/page/year: 6,273,1980
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
1250 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold
-
REFERENCE :
-
HOIZAK Hokkaido Igaku Zasshi. Hokkaido Journal of Medical Science. (Hokkaido Daigaku Igakubu, Nishi-7-chome, Kita-15-jo, Kita-ku, Sapporo, Japan) V.1- 1923- Volume(issue)/page/year: 53,15,1978
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
41 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold Behavioral - ataxia
-
REFERENCE :
-
HOIZAK Hokkaido Igaku Zasshi. Hokkaido Journal of Medical Science. (Hokkaido Daigaku Igakubu, Nishi-7-chome, Kita-15-jo, Kita-ku, Sapporo, Japan) V.1- 1923- Volume(issue)/page/year: 53,15,1978 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
13500 mg/kg/30D-I
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Kidney, Ureter, Bladder - changes in bladder weight Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol)
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 5,353,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
27300 mg/kg/39W-I
-
TOXIC EFFECTS :
-
Cardiac - changes in heart weight Kidney, Ureter, Bladder - other changes in urine composition Nutritional and Gross Metabolic - changes in potassium
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 6,37,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
21600 mg/kg/13W-I
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Kidney, Ureter, Bladder - urine volume increased Blood - changes in leukocyte (WBC) count
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 21,6314,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
2730 mg/kg/13W-I
-
TOXIC EFFECTS :
-
Gastrointestinal - changes in structure or function of salivary glands Gastrointestinal - hypermotility, diarrhea Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
JZKEDZ Jitchuken Zenrinsho Kenkyuho. Central Institute for Experimental Animals, Research Reports. (Jikken Dobutsu Chuo Kenkyusho, 1433 Nogawa, Takatsu-ku, Kawasaki 211, Japan) V.1- 1975- Volume(issue)/page/year: 6,273,1980 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
600 ug/kg
-
SEX/DURATION :
-
female 35 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system Reproductive - Specific Developmental Abnormalities - respiratory system Reproductive - Effects on Newborn - Apgar score (human only)
-
REFERENCE :
-
AJPEEK American Journal of Perinatology. (Thieme Medical Publishers, Inc. 381 Park Ave. S., New York, NY 10157-0208) V.1- 1983- Volume(issue)/page/year: 11,91,1994
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
3150 mg/kg
-
SEX/DURATION :
-
male 63 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - testes, epididymis, sperm duct Reproductive - Fertility - mating performance (e.g. # sperm positive females per # females mated; # copulations per # estrus cycles)
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 9,839,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1 gm/kg
-
SEX/DURATION :
-
female 17-22 day(s) after conception lactating female 14 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 9,879,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
3850 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - extra-embryonic structures (e.g., placenta, umbilical cord) Reproductive - Effects on Embryo or Fetus - fetal death
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 9,851,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1650 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 9,851,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
550 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 9,851,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1800 mg/kg
-
SEX/DURATION :
-
female 17-22 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - stillbirth
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 9,879,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
100 mg/kg
-
SEX/DURATION :
-
female 7-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - other neonatal measures or effects Reproductive - Effects on Newborn - biochemical and metabolic
-
REFERENCE :
-
AIPBE4 Archives Internationalos de Physiologie, de Biochimie et de Biophysique. (Vaillant-Carmanne, s.a., Zeven Puttenstraat 20, 3690 Zutendaal, Belgium) V.99- 1991- Volume(issue)/page/year: 100,355,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
650 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 9,869,1981 *** REVIEWS *** TOXICOLOGY REVIEW PBPSDY Pharmacological and Biochemical Properties of Drug Substances. (American Pharmaceutical Assoc., 2215 Constitution Ave., NW, Washington, DC 20037) V.1- 1977- Volume(issue)/page/year: 2,229,1979
|