1161002-05-6
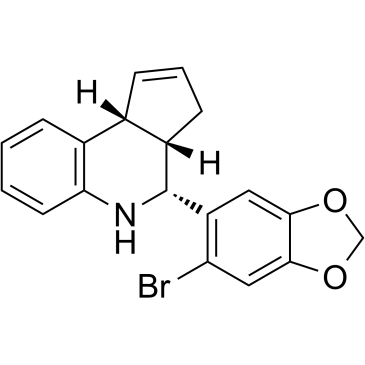
| Name | (3aR,4R,9bS)-4-(6-bromo-1,3-benzodioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline |
|---|---|
| Synonyms |
4-(6-Bromo-benzo[1,3]dioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline
4-(6-Bromo-1,3-benzodioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline |
| Description | G15 is a high affinity and selective G-protein-coupled estrogen receptor (GPER) antagonist with a Ki of 20 nM[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Ki: 20 nM (G-protein-coupled estrogen receptor)[2] |
| In Vitro | G15 (0.1-10 μM; 2 days) inhibits GPER-mediated proliferation stimulated by 17β-estradiol (E2) in A549 and H1793 cell lines[1]. G15 (1 μM; 48 hours) inhibits the response of GPER stimulated by E2 and G1 in A549 and H1793 cell lines[1]. Cell Proliferation Assay[1] Cell Line: A549, H1793 cell lines Concentration: 0.1, 1, 10 μM (combination with 10 nM E2) Incubation Time: 2 days Result: Inhibited GPER-mediated proliferation stimulated by E2. Western Blot Analysis[1] Cell Line: A549, H1793 cell lines Concentration: 1 μM (combination with 10 nM E2 and 10 nM G1) Incubation Time: 48 hours Result: Inhibited the response of GPER stimulated by E2 and G1. |
| In Vivo | G15 (1.46 mg/kg; i.h.; twice a week for 14 weeks) decreases the number of tumor nodules and tumor index increased by the E2 or G1 group in urethane-induced adenocarcinoma mice[1]. Animal Model: Four-week-old female Kunming mice (Urethane-induced adenocarcinoma)[1] Dosage: 1.46 mg/kg (combination with E2, 0.09 mg/kg and fulvestrant (Ful), 2.4 mg/kg) Administration: Subcutaneous injection; twice a week for 14 weeks Result: The number of tumor nodules decreased in the E2+Ful+G15 group. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 462.7±45.0 °C at 760 mmHg |
| Molecular Formula | C19H16BrNO2 |
| Molecular Weight | 370.24 |
| Flash Point | 233.7±28.7 °C |
| Exact Mass | 369.036438 |
| PSA | 30.49000 |
| LogP | 4.49 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.649 |