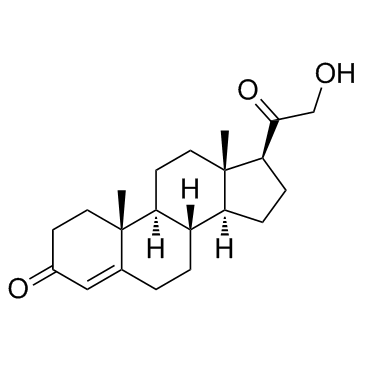
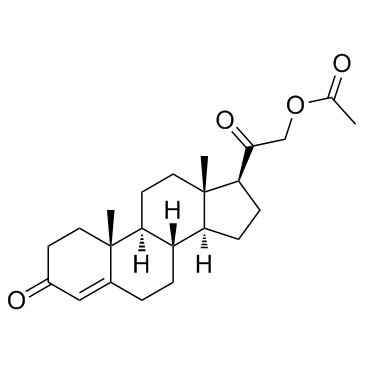
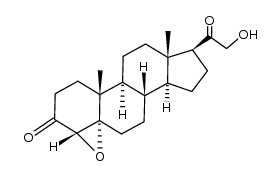
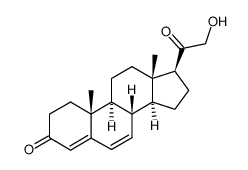
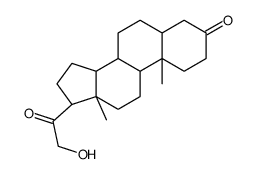
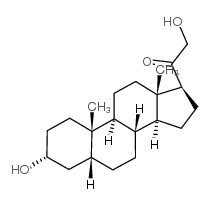
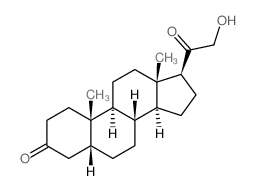
303-01-5
| Name | 5β-dihydrodeoxycorticosterone |
|---|---|
| Synonyms |
21-hydroxy-5|A-pregnan-3,20-dion
21-Hydroxy-5beta-pregnan-3,20-dion hydroxydione 21-hydroxy-4-pregnen-3,20-dione 5beta-dihydrodeoxycorticosterone (5R,8R,9S,10S,13S,14S,17S)-17-(2-hydroxyacetyl)-10,13-dimethyl-1,2,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydrocyclopenta[a]phenanthren-3-one |
| Description | Hydroxydione has an effect of general anesthetic. Hydroxydione is a neuroactive steroid it can be used for anaesthesia related research[1][2]. |
|---|---|
| Related Catalog | |
| In Vivo | Hydroxydione increases the duration of hexobarbital narcosis and repeats hexobarbital doses decrease the duration of hydroxydione narcosis[1]. Hydroxydione (5-100 mg/kg; i.v. once) produces a transient hypotension with little bradycardia and affects the respiration[2]. Animal Model: Cats with chloralose 60 to 80 mg/kg[2] Dosage: 5-100 mg/kg Administration: Intravenous injection; 5-100 mg/kg once Result: Produce severe respiratory depression, but showed no effect on the tibialis in response to excitation of its motor nerve under the condition of chloralose. Produced the usual substantial sustained hypotension seen in chloralosed cats to response the nictitating membrane topreganglionic excitation with a dose of 10 mg/kg. Repressed the respiration in chloralosed cats after a rapid injection. |
| Density | 1.11g/cm3 |
|---|---|
| Boiling Point | 468.6ºC at 760 mmHg |
| Molecular Formula | C21H32O3 |
| Molecular Weight | 332.47700 |
| Flash Point | 251.3ºC |
| Exact Mass | 332.23500 |
| PSA | 54.37000 |
| LogP | 3.77580 |
| Index of Refraction | 1.531 |
|
~% 
303-01-5 |
| Literature: Gelbart,A.; Thomas,R. Journal of Medicinal Chemistry, 1978 , vol. 21, p. 284 - 288 |
|
~28% 
303-01-5 |
| Literature: Kluge; Schulz; Liebsch Tetrahedron, 1996 , vol. 52, # 8 p. 2957 - 2976 |
|
~% 
303-01-5 |
| Literature: Upjohn Co. Patent: US2817670 , 1952 ; |
|
~% 
303-01-5 |
| Literature: Upjohn Co. Patent: US2817670 , 1952 ; |