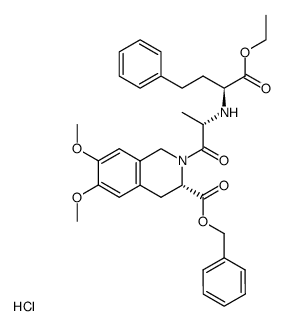
82586-52-5
| Name | (3S)-2-[(2S)-2-[[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]propanoyl]-6,7-dimethoxy-3,4-dihydro-1H-isoquinoline-3-carboxylic acid,hydrochloride |
|---|---|
| Synonyms |
Uniretic
Univasc SPM 925 Moexipril HCl Moexipril HCL MOEXIPRIL HYDROCHLORIDE CI-925 UNII-Q1UMG3UH45 Moexipril (hydrochloride) |
| Description | Moexipril HCl is a potent orally active non-sulfhydryl angiotensin converting enzyme(ACE) inhibitor, which is used for the treatment of hypertension and congestive heart failure. Target: ACEMoexipril is a long-acting ACE inhibitor suitable for once-daily administration, and like some ACE inhibitors, moexipril is a prodrug and needs to be hydrolyzed in the liver into its active carboxylic metabolite, moexiprilat, to become effective [1]. Upon oral administration of moexipril (10 mg/kg/day) to spontaneously hypertensive rats, plasma angiotensin II concentration decreased to undetectable levels, plasma ACE activity was inhibited by 98% and plasma angiotensin I concentration increased 8.6-fold 1 h after dosing. At 24 h, plasma angiotensin I and angiotensin II concentrations had returned to pretreatment levels, whereas plasma ACE activity was still inhibited by 56%. Four-week oral administration of moexipril (0.1-30 mg/kg/day) to spontaneously hypertensive rats lowered blood pressure and differentially inhibited ACE activity in plasma, lung, aorta, heart and kidney in a dose-dependent fashion [2, 3]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 709.3ºC at 760 mmHg |
|---|---|
| Melting Point | 141-161ºC |
| Molecular Formula | C27H35ClN2O7 |
| Molecular Weight | 535.03 |
| Flash Point | 382.8ºC |
| PSA | 114.40000 |
| LogP | 3.71510 |
| Storage condition | 2-8°C |
| Symbol |

GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H400 |
| Precautionary Statements | P273 |
| Personal Protective Equipment | Eyeshields;Gloves |
| Hazard Codes | N:Dangerous for the environment |
| Risk Phrases | R50 |
| Safety Phrases | 60 |
| RIDADR | UN 3077 |
| RTECS | NW7174500 |
|
~88% 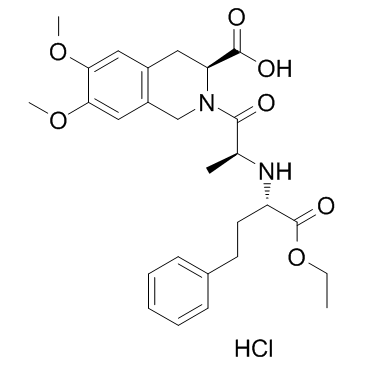
82586-52-5 |
| Literature: Klutchko; Blankley; Fleming; Hinkley; Werner; Nordin; Holmes; Hoefle; Cohen; Essenburg Journal of Medicinal Chemistry, 1986 , vol. 29, # 10 p. 1953 - 1961 |
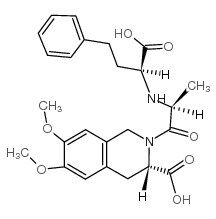
| Precursor 1 | |
|---|---|
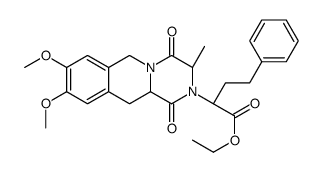
| DownStream 2 | |