55224-94-7
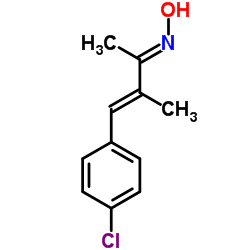
| Name | 4-(4-Chlorophenyl)-3-methyl-3-buten-2-oneoxime |
|---|---|
| Synonyms |
(2E,3E)-4-(4-Chlorophenyl)-N-hydroxy-3-methyl-3-buten-2-imine
(2E,3E)-4-(4-Chlorophenyl)-N-hydroxy-3-methylbut-3-en-2-imine N-Desmethylclozapine 4-(4-chlorophenyl)-3-methylbut-3-en-2-one oxime AP-18 |
| Description | AP-18, a potent and selective TRPA1 inhibitor, blocks activation of TRPA1 by 50 μM Cinnamaldehyde with an IC50 of 3.1 μM and 4.5 μM for human and mouse TRPA1, respectively. AP-18 reverses complete Freund's adjuvant (CFA)-induced mechanical hyperalgesia in mice. AP-18 attenuated 30 μM AITC-induced Yo-Pro uptake in a concentration-dependent manner, with an IC50 of 10.3 μM[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
TRPA1:~3 μM (IC50) |
| In Vitro | At concentrations up to 50 μM, AP-18 is unable to appreciably block activation of TRPV1, TRPV2, TRPV3, TRPV4, or TRPM8. AP-18 reversibly blocks mouse TRPA1 responses to iodoacetamide (an irreversible cysteine-alkylating agent) in CHO cells assayed by ratiometric Ca2+ imaging. AP-18 also blocks cold- and mustard-oil-induced activation of mouse TRPA1. AP-18 blocks cinnamaldehyde-induced TRPA1 currents in excised patches from Xenopus oocytes[1]. |
| In Vivo | AP18 (1 mM; injected in hindpaw of mice) significantly blocks cinnamaldehdye-induced but not capsaicin-induced nociceptive events, demonstrating efficacy and specificity[1]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 348.4±34.0 °C at 760 mmHg |
| Molecular Formula | C11H12ClNO |
| Molecular Weight | 209.672 |
| Flash Point | 164.5±25.7 °C |
| Exact Mass | 209.060745 |
| PSA | 32.59000 |
| LogP | 3.72 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.529 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | 22 |
| RIDADR | NONH for all modes of transport |