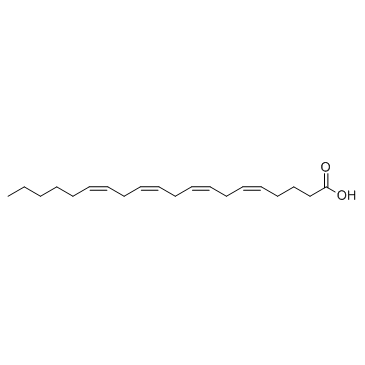
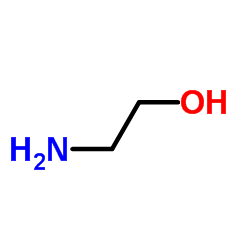
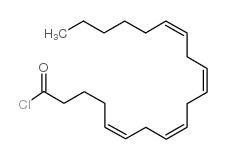
94421-68-8
| Name | anandamide |
|---|---|
| Synonyms |
arachidonic acid ethanolamide
MFCD00153766 (5Z,8Z,11Z,14Z)-N-(2-Hydroxyethyl)-5,8,11,14-icosatetraenamide N-arachidonoylethanolamide N-arachidonoylethanolamine arachidonylethanolamide 5,8,11,14-Eicosatetraenoylethanolamide N-Arachidonoyl-2-hydroxyethylamide N-arachidonoyl ethanolamine arachidonoylethanolamide Arachidonoyl-EA (5Z,8Z,11Z,14Z)-N-(2-Hydroxyethyl)icosa-5,8,11,14-tetraenamide |
| Description | Anandamide is an immune modulator in the central nervous system acts via not only cannabinoid receptors (CB1 and CB2) but also other targets (e.g., GPR18/GPR55). |
|---|---|
| Related Catalog | |
| Target |
CB1 Receptor CB2 Receptor GPR18/GPR55 Human Endogenous Metabolite |
| In Vitro | Anandamide, acting via CB2 receptors, alleviates lipopolysaccharide (LPS)-induced neuroinflammation in rat primary microglial cultures. The endocannabinoid system modulates both neuronal and immune functions through two protein-coupled cannabinoid receptors (CB1 and CB2), although endocannabinoids, especially Anandamide (AEA), can activate numerous other receptors like PPARS, TRPV1, and GPR18/GPR55[1]. |
| In Vivo | Anandamide is an endocannabinoid binding both CB1R and CB2R. To evaluate the impact of CBR activation on whole-body glucose homeostasis, glucose tolerance is assessed after a single intraperitoneal Anandamide injection (10 mg/kg). The increase in glycemia in response to glucose ingestion is considerably smaller in mice treated with Anandamide compared with control, and this is associated with an improvement of glucose tolerance as illustrated by the AUC0-2h calculations[2]. |
| Animal Admin | Mice[2] Eleven-week-old C57BL/6J male mice and global CB1R-/- mice are housed individually on a 12/12-h light/dark schedule at 22-23°C with ad libitum access to water and food. A group of mice is subject to a high-fat diet (30% lard). After 16 weeks of diet, animals with a weight gain less than +10 g compared with controls are excluded from the study. Diet-induced obesity (DIO) mice (39.1±1.1 vs. 27.3±0.9 g, DIO vs. control) are glucose intolerant and insulin resistant. On the day of each experiment, food is removed from the cages for 6 h (from 8:00 A.M. to 2:00 P.M.). Anandamide is administered intraperitoneally at 10 mg/kg. In control experiments, animals are injected with vehicle (4% DMSO/1% Tween 80)[2]. |
| References |
| Density | 0.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 522.3±50.0 °C at 760 mmHg |
| Molecular Formula | C22H37NO2 |
| Molecular Weight | 347.535 |
| Flash Point | 269.7±30.1 °C |
| Exact Mass | 347.282440 |
| PSA | 49.33000 |
| LogP | 5.66 |
| Vapour Pressure | 0.0±3.1 mmHg at 25°C |
| Index of Refraction | 1.504 |
| Storage condition | −20°C |
| Water Solubility | ethanol: soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | F: Flammable; |
| Risk Phrases | R11 |
| Safety Phrases | 24/25-16-7 |
| RIDADR | UN 1170 3 |
| WGK Germany | 3 |
| RTECS | JX3842500 |
| HS Code | 2924199090 |
|
~85% 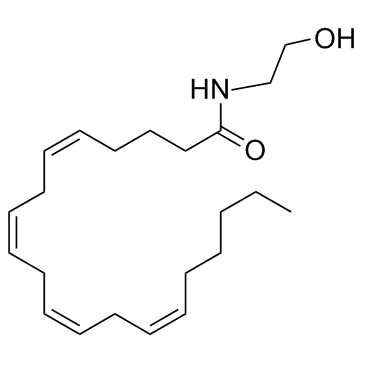
94421-68-8 |
| Literature: Ottria, Roberta; Casati, Silvana; Ciuffreda, Pierangela Chemistry and Physics of Lipids, 2012 , vol. 165, # 7 p. 705 - 711 |
|
~71% 
94421-68-8 |
| Literature: Ferreri, Carla; Anagnostopoulos, Dimitris; Lykakis, Ioannis N.; Chatgilialoglu, Chryssostomos; Siafaka-Kapadai, Athanassia Bioorganic and Medicinal Chemistry, 2008 , vol. 16, # 18 p. 8359 - 8365 |
|
~% 
94421-68-8 |
| Literature: US5925678 A1, ; |
|
~91% 
94421-68-8 |
| Literature: Adams, Irma B.; Ryan, William; Singer, Michael; Razdan, Raj K.; Compton, David R.; Martin, Billy R. Life Sciences, 1995 , vol. 56, # 23-24 p. 2041 - 2048 |
|
~% 
94421-68-8 |
| Literature: Biochemical Pharmacology, , vol. 69, # 8 p. 1187 - 1193 |
|
~% 
94421-68-8 |
| Literature: wyffels, Leonie; De Bruyne, Sylvie; Blanckaert, Peter; Lambert, Didier M.; De Vos, Filip Bioorganic and Medicinal Chemistry, 2009 , vol. 17, # 1 p. 49 - 56 |
|
~% 
94421-68-8 |
| Literature: Biochemical Pharmacology, , vol. 53, # 3 p. 255 - 260 |
| Precursor 7 | |
|---|---|
| DownStream 0 | |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |